New approach methodologies in human regulatory toxicology – Not if, but how and when! - ScienceDirect
Por um escritor misterioso
Last updated 26 março 2025

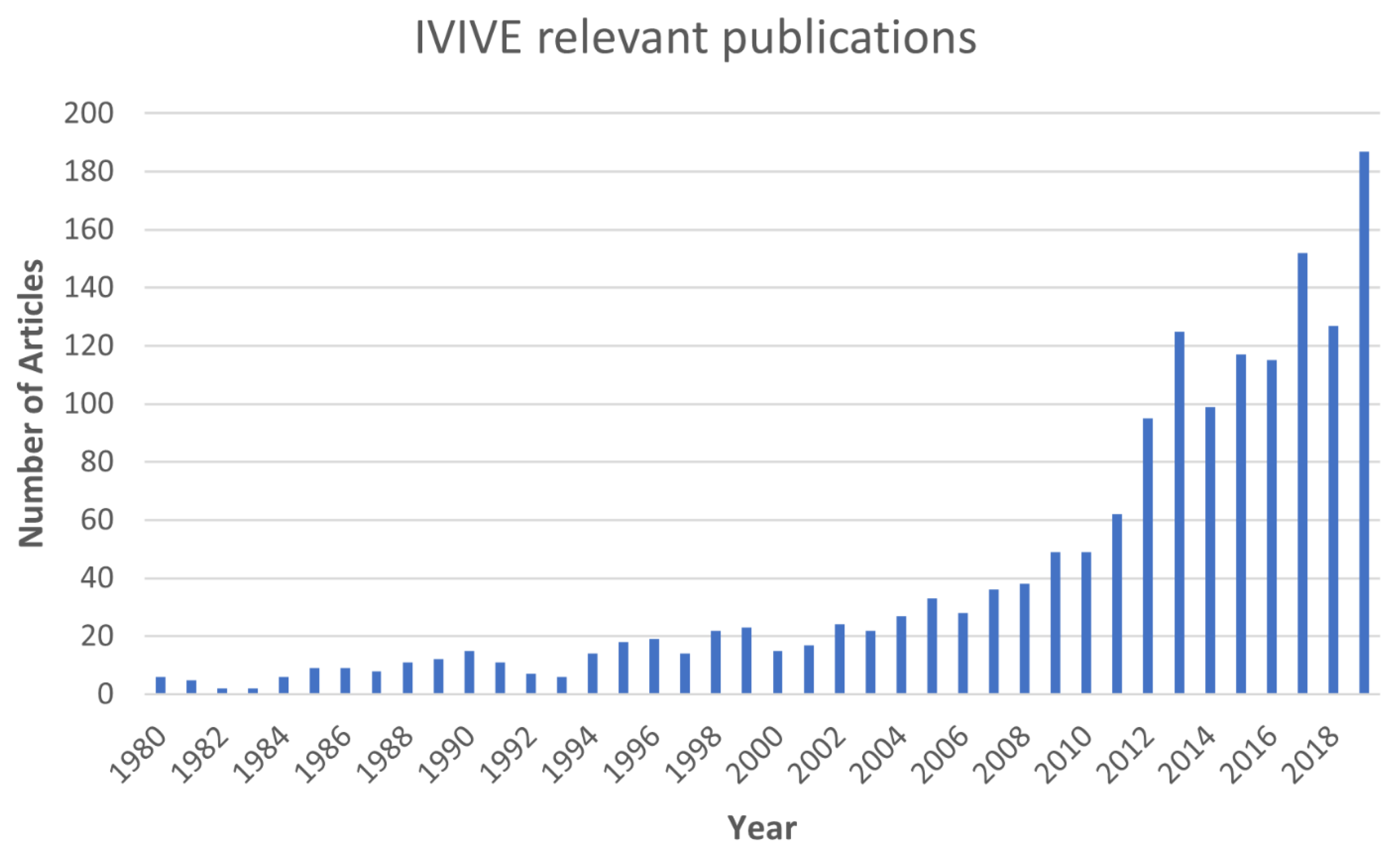
Toxics, Free Full-Text

Envisioning an international validation process for New Approach Methodologies in chemical hazard and risk assessment - ScienceDirect

Limitations of Animal Studies for Predicting Toxicity in Clinical Trials: Is it Time to Rethink Our Current Approach? - ScienceDirect

Job-Related Problems Prior to Nurse Suicide, 2003-2017: A Mixed Methods Analysis Using Natural Language Processing and Thematic Analysis - Journal of Nursing Regulation

PDF) Combination of computational new approach methodologies for enhancing evidence of biological pathway conservation across species
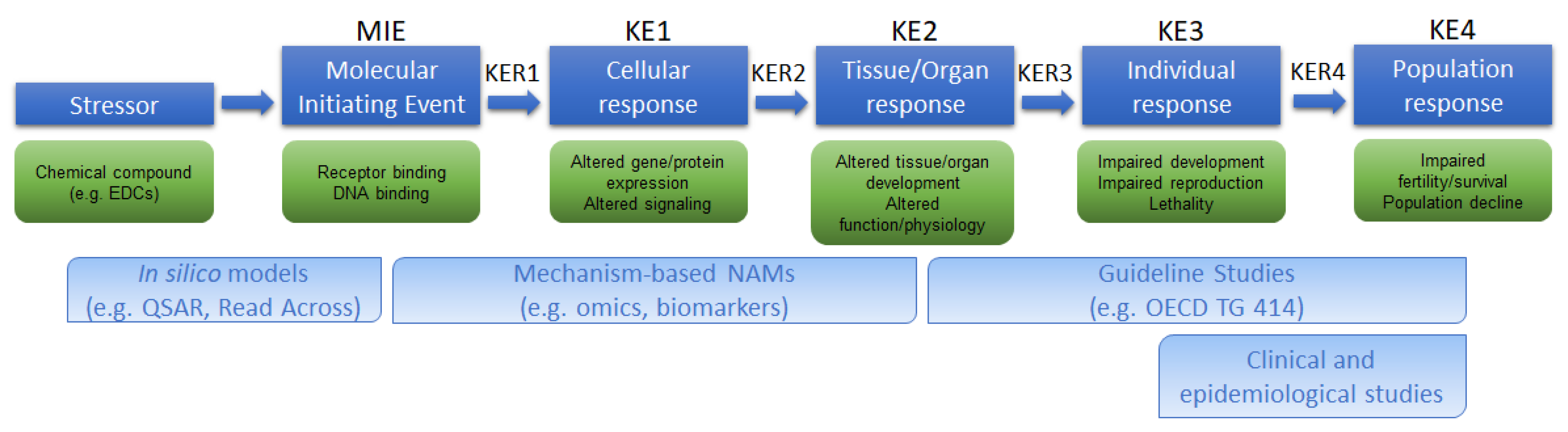
IJERPH, Free Full-Text

Modernizing Human Cancer Risk Assessment of Therapeutics: Trends in Pharmacological Sciences

Japan-Specific Key Regulatory Aspects for Development of New Biopharmaceutical Drug Products - Journal of Pharmaceutical Sciences

Distinguishing between expert and statistical systems for application under ICH M7 - ScienceDirect

Principles underpinning the use of new methodologies in the risk assessment of cosmetic ingredients - ScienceDirect

Strategies to improve the regulatory assessment of developmental neurotoxicity (DNT) using in vitro methods - ScienceDirect
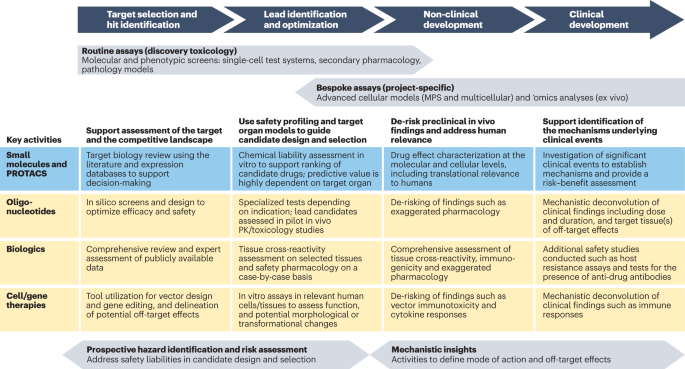
The evolving role of investigative toxicology in the pharmaceutical industry

Human risk associated with exposure to mixtures of antiandrogenic chemicals evaluated using in vitro hazard and human biomonitoring data - ScienceDirect

Comparison of regulatory pathways for the approval of advanced therapies in the European Union and the United States - Cytotherapy
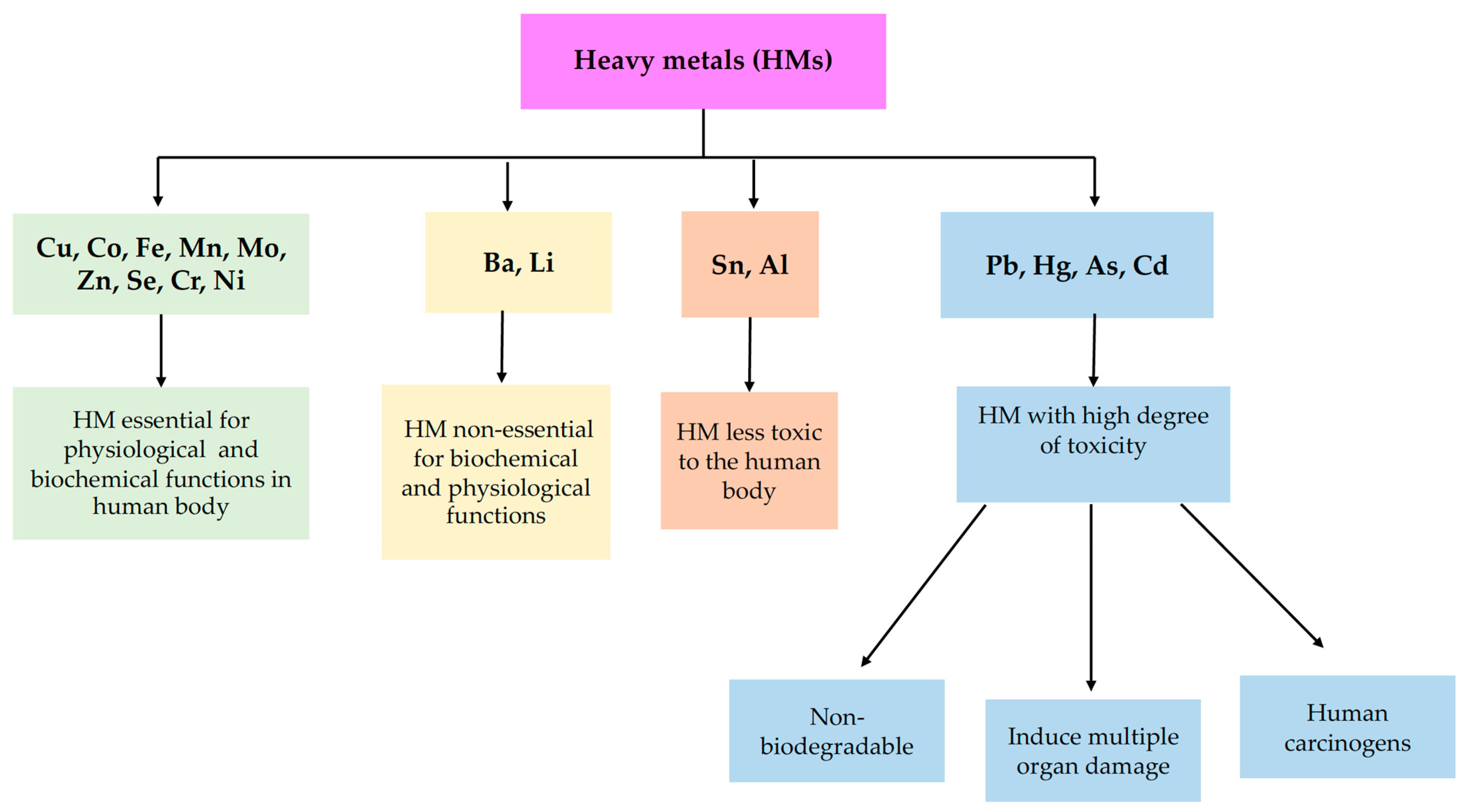
Toxics, Free Full-Text
Recomendado para você
-
 Press Archive - Stanford Law School26 março 2025
Press Archive - Stanford Law School26 março 2025 -
 Kelly Godoy - 03.04.201526 março 2025
Kelly Godoy - 03.04.201526 março 2025 -
 Kelly Godoy (@KellyGodoy) / X26 março 2025
Kelly Godoy (@KellyGodoy) / X26 março 2025 -
 Record News completa 15 anos com nova identidade visual e apresenta crescimento de audiência de 150%26 março 2025
Record News completa 15 anos com nova identidade visual e apresenta crescimento de audiência de 150%26 março 2025 -
 Tudo sobre Kelly Godoy - Portal Alta Definição26 março 2025
Tudo sobre Kelly Godoy - Portal Alta Definição26 março 2025 -
 Ariana Godoy - Age, Family, Bio26 março 2025
Ariana Godoy - Age, Family, Bio26 março 2025 -
 Season 4, Ceazar Chan Wiki26 março 2025
Season 4, Ceazar Chan Wiki26 março 2025 -
 Elizabeth Holmes - Wikipedia26 março 2025
Elizabeth Holmes - Wikipedia26 março 2025 -
 B Baker - LongeviQuest26 março 2025
B Baker - LongeviQuest26 março 2025 -
 Who is Ariana Godoy? Wiki, Biography, Net worth, Husband, Kids, Family, Age, Height, Ethnicity & More26 março 2025
Who is Ariana Godoy? Wiki, Biography, Net worth, Husband, Kids, Family, Age, Height, Ethnicity & More26 março 2025
você pode gostar
-
 Cama Carro Infantil tamanho juvenil Barbie26 março 2025
Cama Carro Infantil tamanho juvenil Barbie26 março 2025 -
 2Cap GTA 5 Pc Game Download (Offline only) No CD/DVD/Code (Complete Game) (Complete Edition) Price in India - Buy 2Cap GTA 5 Pc Game Download (Offline only) No CD/DVD/Code (Complete Game) (Complete26 março 2025
2Cap GTA 5 Pc Game Download (Offline only) No CD/DVD/Code (Complete Game) (Complete Edition) Price in India - Buy 2Cap GTA 5 Pc Game Download (Offline only) No CD/DVD/Code (Complete Game) (Complete26 março 2025 -
 I was going through some old games and realized, WHERE'S THE REMASTER / PC PORT OF THIS?! 2004 wasn't ready for 3rd person 4 player online coop shooters, but it would be26 março 2025
I was going through some old games and realized, WHERE'S THE REMASTER / PC PORT OF THIS?! 2004 wasn't ready for 3rd person 4 player online coop shooters, but it would be26 março 2025 -
 Pokemon SoulSilver #07 - Resolvendo mistérios de Unown26 março 2025
Pokemon SoulSilver #07 - Resolvendo mistérios de Unown26 março 2025 -
JOGOS DE FOGOS DE ARTIFÍCIO GRÁTIS26 março 2025
-
 I compiled a video 20+ GGST May Combos (simple + advanced), feel free to check it out! : r/Guiltygear26 março 2025
I compiled a video 20+ GGST May Combos (simple + advanced), feel free to check it out! : r/Guiltygear26 março 2025 -
 Making a meme with photoshop26 março 2025
Making a meme with photoshop26 março 2025 -
 Tonikaku Kawaii 2nd Season - Dublado - TONIKAWA: Over The Moon For26 março 2025
Tonikaku Kawaii 2nd Season - Dublado - TONIKAWA: Over The Moon For26 março 2025 -
Associação Leopoldinense de Xadrez - ALEX26 março 2025
-
 Scratch: 2D Minecraft (Advanced) Tutorial (Ep.1)26 março 2025
Scratch: 2D Minecraft (Advanced) Tutorial (Ep.1)26 março 2025
