ASAS-HI improvement ≥30%, ASDAS LDA status and ASAS40 response
Por um escritor misterioso
Last updated 07 abril 2025

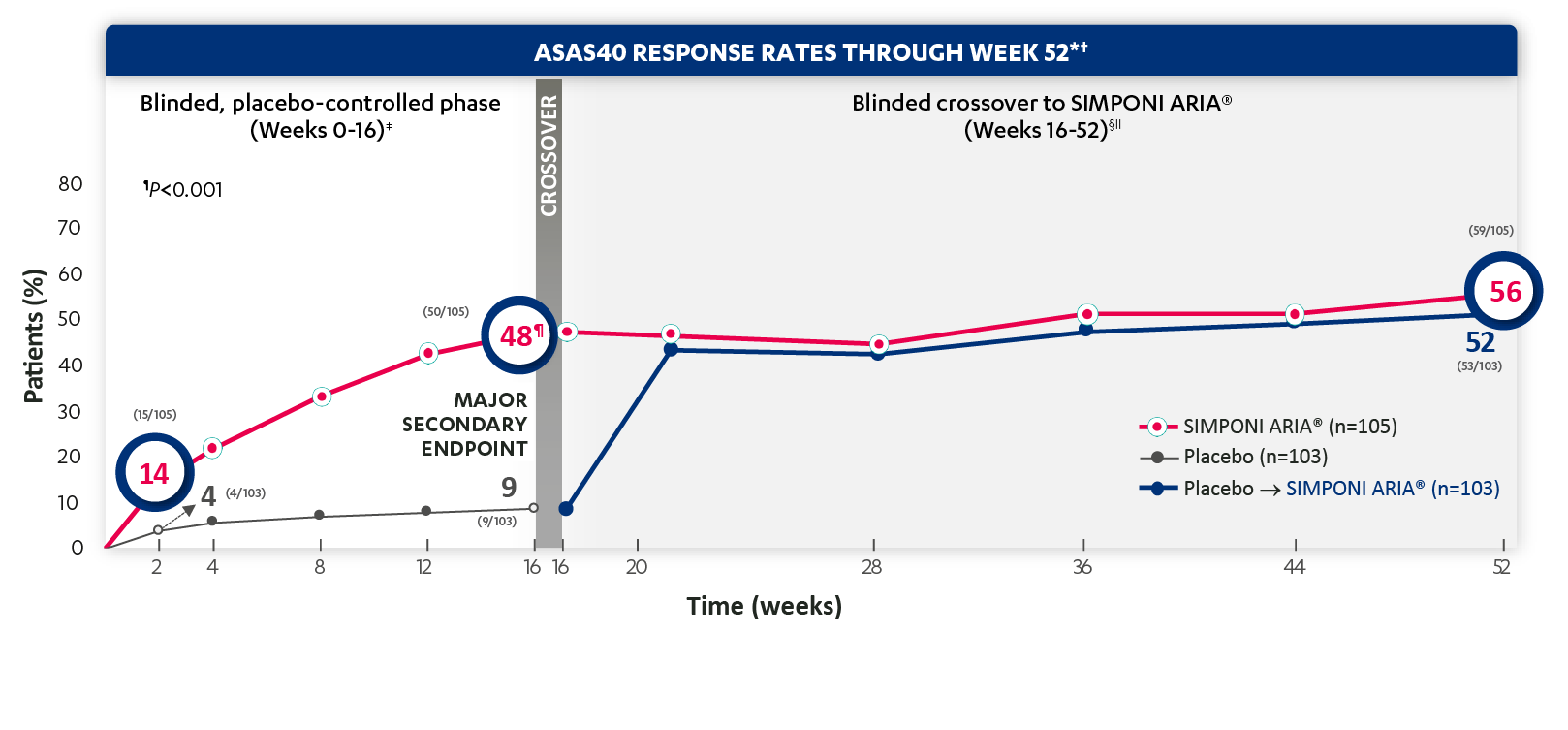
SIMPONI ARIA® Ankylosing Spondylitis: ASAS Response Rates

Efficacy and safety of upadacitinib for active ankylosing spondylitis refractory to biological therapy: a double-blind, randomised, placebo-controlled phase 3 trial
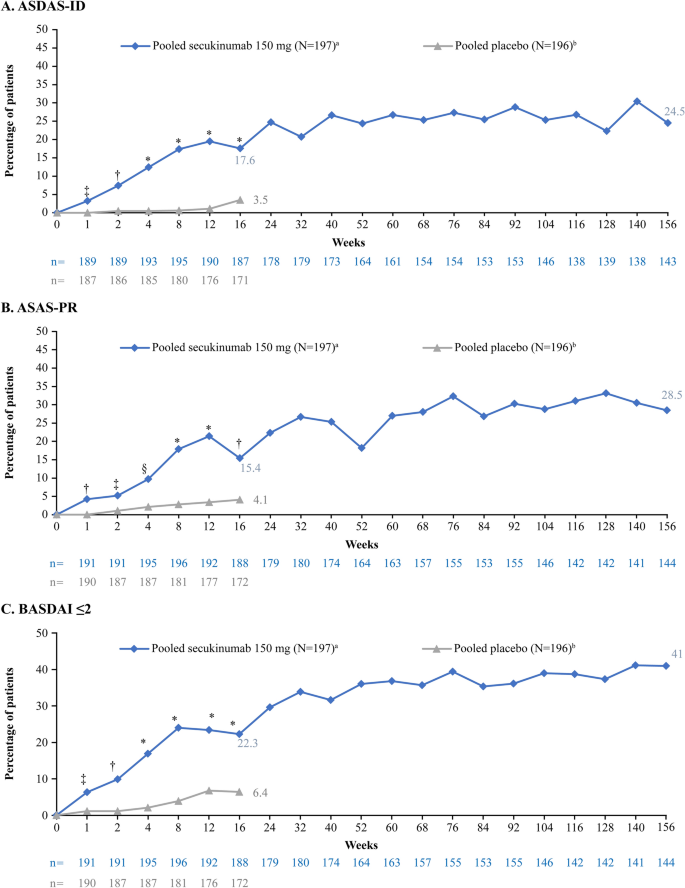
Achievement of Remission Endpoints with Secukinumab Over 3 Years in Active Ankylosing Spondylitis: Pooled Analysis of Two Phase 3 Studies

Treating to Target(s) With Interleukin-17 Inhibitors - Charles W. Lynde, Jennifer Beecker, Jan Dutz, Cathy Flanagan, Lyn C. Guenther, Wayne Gulliver, Kim Papp, Proton Rahman, Dalton Sholter, Gordon E. Searles, 2019

The state of the art—psoriatic arthritis outcome assessment in clinical trials and daily practice - The Lancet Rheumatology

The Sensitivity to Change of the ASAS Health Index in an Observational Real-Life Cohort Study

Efficacy and safety of upadacitinib for active ankylosing spondylitis refractory to biological therapy: a double-blind, randomised, placebo-controlled phase 3 trial. - Abstract - Europe PMC

UCB Presents New Five-Year Data on BIMZELX® (bimekizumab-bkzx) in Ankylosing Spondylitis at ACR Convergence 2023
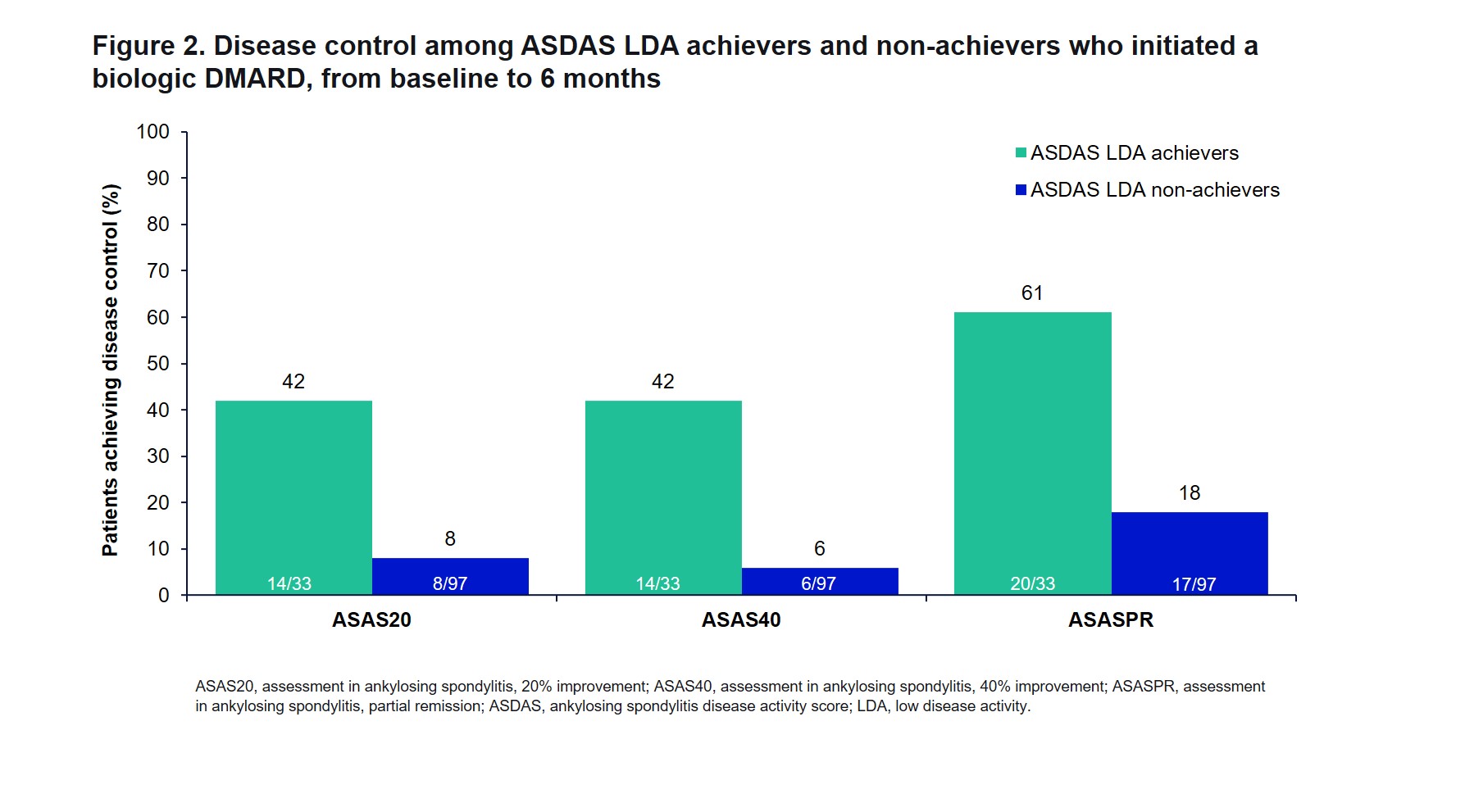
Impact of Achieving ASDAS LDA on Disease Activity and Patient-Reported Outcome Measures Among Patients with Ankylosing Spondylitis Treated with Biologic DMARDs - ACR Meeting Abstracts

PDF) Treatment response and drug retention rates in 24 195 biologic-naïve patients with axial spondyloarthritis initiating TNFi treatment: routine care data from 12 registries in the EuroSpA collaboration

Long-Term Safety and Efficacy of Ixekizumab in Patients With Axial Spondyloarthritis: 3-year Data From the COAST Program
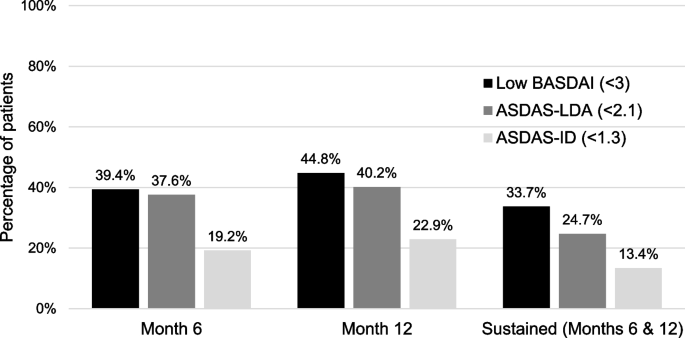
Sustained low functional impairment in axial spondyloarthritis (axSpA): which are the primary outcomes that should be targeted to achieve this?, Arthritis Research & Therapy
Recomendado para você
-
Changes of disease activity [(a) ASDAS; (b) BASDAI] for TNF-α inhibitor07 abril 2025
-
 asdas – myCloudDoor – Expertise for Cloud Transition07 abril 2025
asdas – myCloudDoor – Expertise for Cloud Transition07 abril 2025 -
Asda - asdas Sheet music for Piano (Solo) Easy07 abril 2025
-
 ASDAS Figma Community07 abril 2025
ASDAS Figma Community07 abril 2025 -
 Asdas07 abril 2025
Asdas07 abril 2025 -
 Asda reduces emissions by 16% over 12-month period - edie07 abril 2025
Asda reduces emissions by 16% over 12-month period - edie07 abril 2025 -
Asdas Svg Png Icon Free Download (#77028)07 abril 2025
-
 Asda reveals real reason they use self-checkout cameras and it's not about data - Mirror Online07 abril 2025
Asda reveals real reason they use self-checkout cameras and it's not about data - Mirror Online07 abril 2025 -
 03 Simple equations - asdas - Topic: Simple equations Question: Solve for the variable. x + 5 = 10 - Studocu07 abril 2025
03 Simple equations - asdas - Topic: Simple equations Question: Solve for the variable. x + 5 = 10 - Studocu07 abril 2025 -
 asdas Tapestry Wall Hanging,Polyester Home Decoration Bedroom Living Room Tapestries, Strawberry Rabbit Tablecloth Landscape Art Sofa Background Wall Covering Decoration Gifts Young People,Strawber, 150*130cm : Home & Kitchen07 abril 2025
asdas Tapestry Wall Hanging,Polyester Home Decoration Bedroom Living Room Tapestries, Strawberry Rabbit Tablecloth Landscape Art Sofa Background Wall Covering Decoration Gifts Young People,Strawber, 150*130cm : Home & Kitchen07 abril 2025
você pode gostar
-
 Chessbase Magazine №194: The Magazine for Professional Chess (SDVL) FREE Download07 abril 2025
Chessbase Magazine №194: The Magazine for Professional Chess (SDVL) FREE Download07 abril 2025 -
 Desenhos Online para colorir e imprimir!: Carro de corrida pra pintar07 abril 2025
Desenhos Online para colorir e imprimir!: Carro de corrida pra pintar07 abril 2025 -
 Dusttale Dust!Sans fight(no heal) on Make a GIF07 abril 2025
Dusttale Dust!Sans fight(no heal) on Make a GIF07 abril 2025 -
 CoD Mobile devs break down major Season 2 battle royale changes07 abril 2025
CoD Mobile devs break down major Season 2 battle royale changes07 abril 2025 -
 Mousehouse Jack Cheese (Exclusive!)07 abril 2025
Mousehouse Jack Cheese (Exclusive!)07 abril 2025 -
Marcos Uchôa sai em defesa da convocação de Dani Alves para a Copa do Mundo - Vídeo Dailymotion07 abril 2025
-
 Super KIT QUEBRA-CABEÇA Domino e Jogo da Memoria Disney Princesas JAK 2354 – Starhouse Mega Store07 abril 2025
Super KIT QUEBRA-CABEÇA Domino e Jogo da Memoria Disney Princesas JAK 2354 – Starhouse Mega Store07 abril 2025 -
 One Piece: Chopper's Kingdom on the Island of Strange Animals07 abril 2025
One Piece: Chopper's Kingdom on the Island of Strange Animals07 abril 2025 -
[AnimeFire.net] Tokyo Revengers (Dublado) - Episódio 1 (HD).mp407 abril 2025
-
fifa 19 xbox 36007 abril 2025


