Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV
Por um escritor misterioso
Last updated 11 abril 2025


COVID-19 Vaccine Frontrunners and Their Nanotechnology Design

Immunogenicity of clinically relevant SARS-CoV-2 vaccines in nonhuman primates and humans
DNA vaccine candidate encoding SARS-CoV-2 spike proteins elicited potent humoral and Th1 cell-mediated immune responses in mice

An Update on the Status of Vaccine Development for SARS-CoV-2 Including Variants. Practical Considerations for COVID-19 Special Populations - Bulent Kantarcioglu, Omer Iqbal, Joseph Lewis, Charles A. Carter, Meharvan Singh, Fabio Lievano

Design, immunogenicity and efficacy of a Pan-SARS-CoV-2 synthetic DNA vaccine
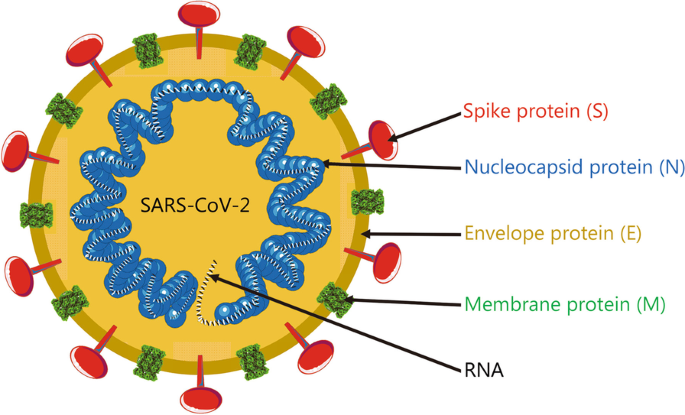
Advances in the design and development of SARS-CoV-2 vaccines, Military Medical Research

704/DNA vaccines leverage cytoplasmic DNA stimulation to promote anti-HIV neutralizing antibody production in mice and strong immune response against alpha-fetoprotein in non-human primates: Molecular Therapy - Nucleic Acids

Safety, tolerability, and immunogenicity of a SARS-CoV-2 recombinant spike RBD protein vaccine: A randomised, double-blind, placebo-controlled, phase 1-2 clinical trial (ABDALA Study) - eClinicalMedicine

INOVIO COVID-19 Vaccine Trial Update - December 2020 - Penn Medicine

An Update on the Status of Vaccine Development for SARS-CoV-2 Including Variants. Practical Considerations for COVID-19 Special Populations - Bulent Kantarcioglu, Omer Iqbal, Joseph Lewis, Charles A. Carter, Meharvan Singh, Fabio Lievano
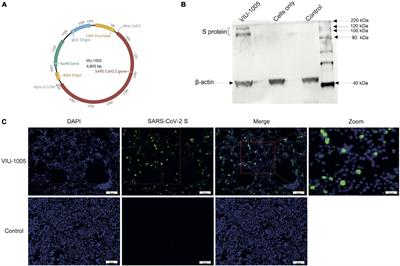
Frontiers Synthetic SARS-CoV-2 Spike-Based DNA Vaccine Elicits Robust and Long-Lasting Th1 Humoral and Cellular Immunity in Mice

Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV-2: A preliminary report of an open-label, Phase 1 clinical trial - eClinicalMedicine

Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV-2: A preliminary report of an open-label, Phase 1 clinical trial - eClinicalMedicine
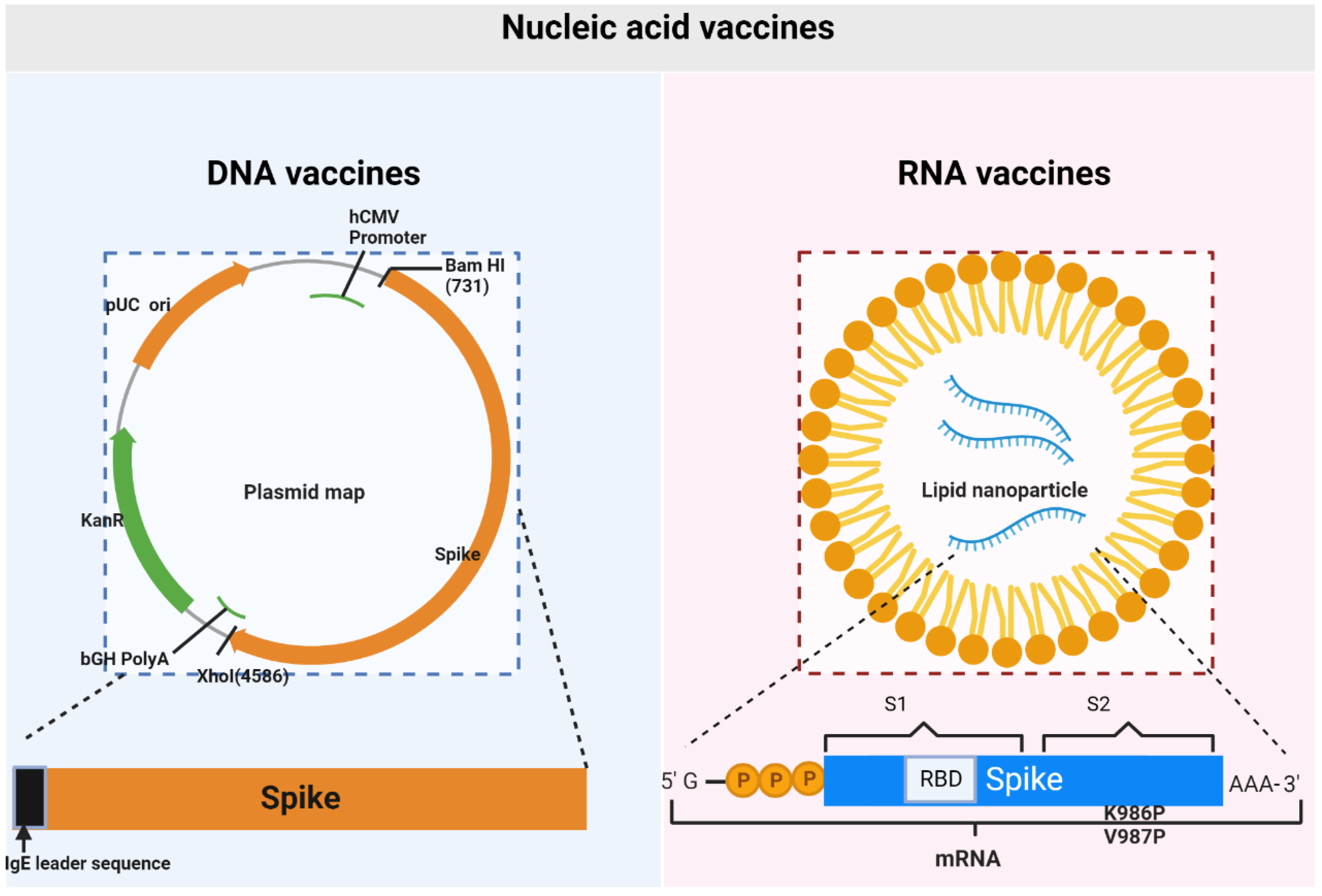
Vaccines, Free Full-Text

Delving into revolutionary SARS-CoV-2 vaccine approaches: Molecular and virological examinations; principles of SARS-CoV-2 vaccine platform
Recomendado para você
-
 Binance Dual Investment FREE ETH QUIZ ANSWERS!11 abril 2025
Binance Dual Investment FREE ETH QUIZ ANSWERS!11 abril 2025 -
 Binance Dual Investment - Learn & Earn Survey Answers.11 abril 2025
Binance Dual Investment - Learn & Earn Survey Answers.11 abril 2025 -
 Public Technology Institute (PTI) • Fusion Learning Partners11 abril 2025
Public Technology Institute (PTI) • Fusion Learning Partners11 abril 2025 -
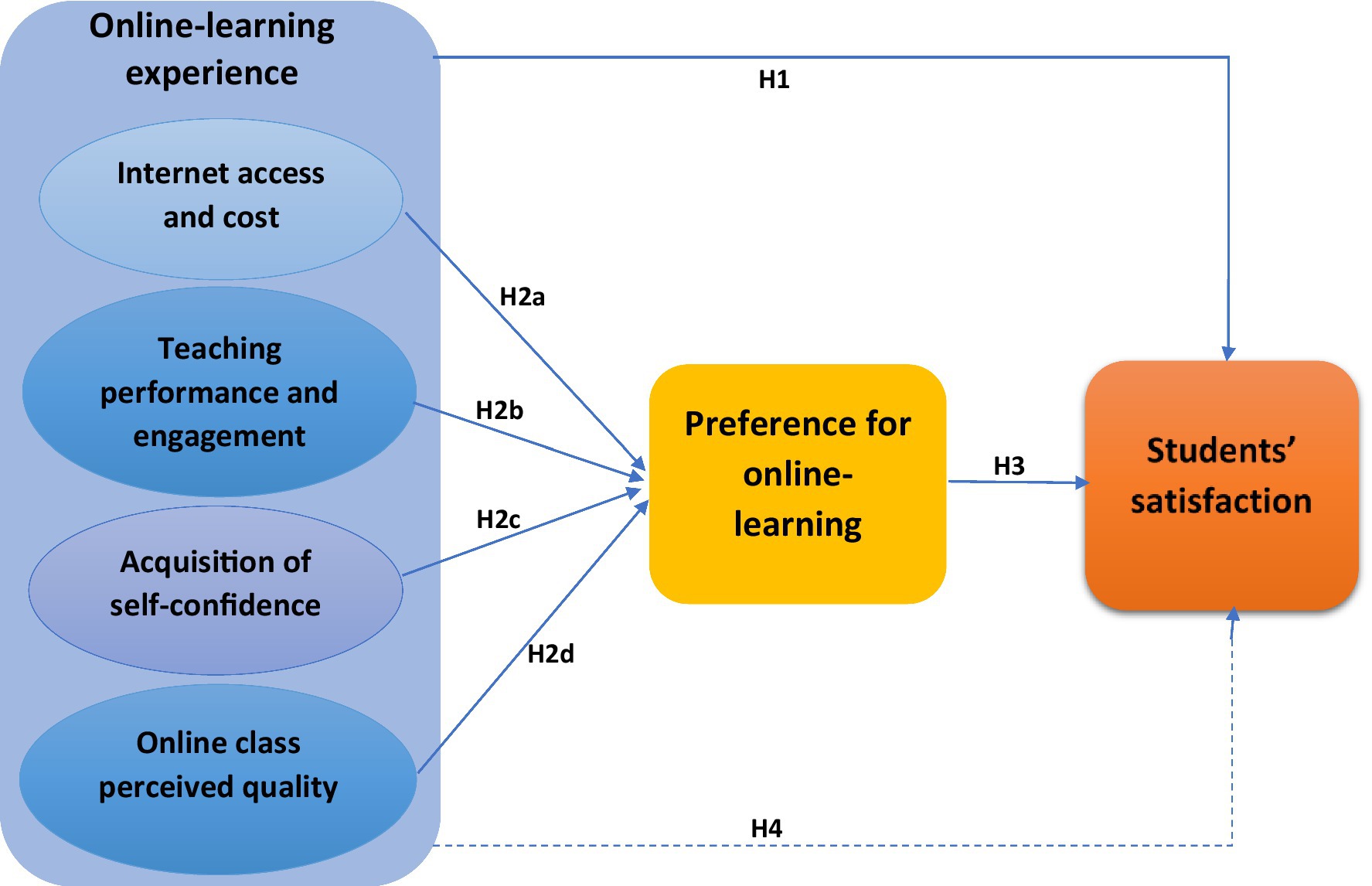 Frontiers The effect of students' online learning experience on11 abril 2025
Frontiers The effect of students' online learning experience on11 abril 2025 -
 The 9 Box Grid: How to Use It, Practical Template, And11 abril 2025
The 9 Box Grid: How to Use It, Practical Template, And11 abril 2025 -
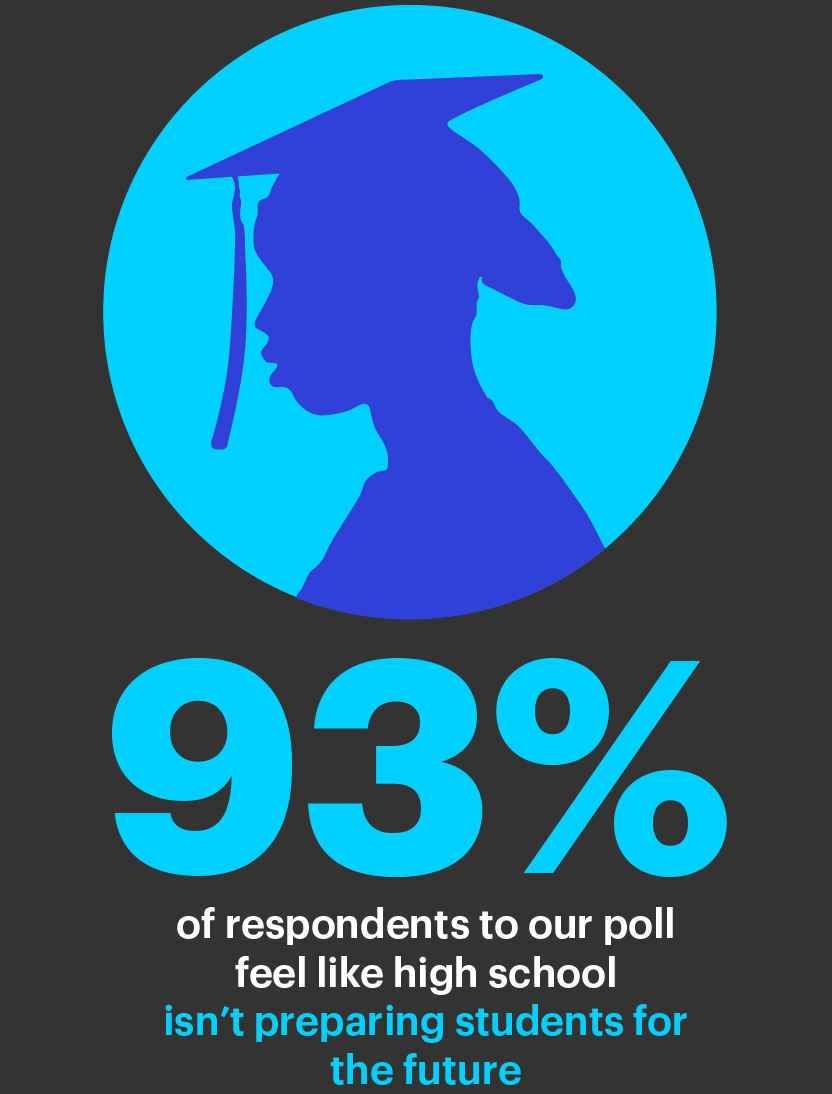 Are High Schools Preparing Students For The Future - XQ11 abril 2025
Are High Schools Preparing Students For The Future - XQ11 abril 2025 -
Complete each questionnaire entirely by answering11 abril 2025
-
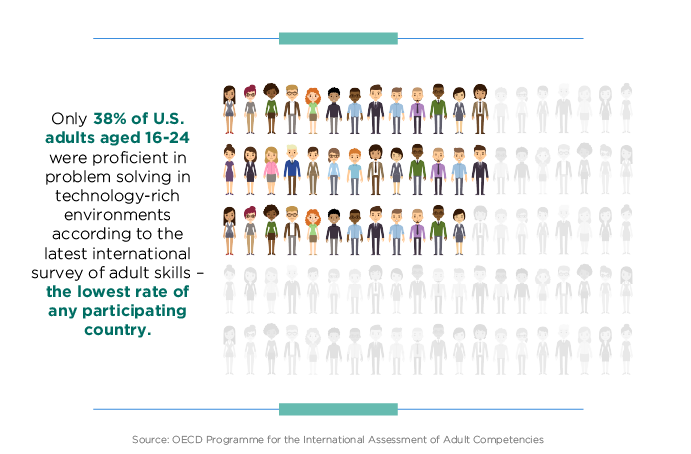 Blueprint - NCEE11 abril 2025
Blueprint - NCEE11 abril 2025 -
 Dual use of e-cigarettes with conventional tobacco is associated11 abril 2025
Dual use of e-cigarettes with conventional tobacco is associated11 abril 2025 -
:max_bytes(150000):strip_icc()/banking.asp-Final-e3a67ff9762b40aeac56983c22695032.jpg) The Evolution of Banking Over Time11 abril 2025
The Evolution of Banking Over Time11 abril 2025
você pode gostar
-
 My Little Pony - Equestria Girls Logo PNG Vector (SVG) Free Download11 abril 2025
My Little Pony - Equestria Girls Logo PNG Vector (SVG) Free Download11 abril 2025 -
 The results of friendly matches of the best football clubs, barcelona, real madrid11 abril 2025
The results of friendly matches of the best football clubs, barcelona, real madrid11 abril 2025 -
 in 2023 Roblox creator, Roblox, Free t shirt design11 abril 2025
in 2023 Roblox creator, Roblox, Free t shirt design11 abril 2025 -
 Pin by EWERTON DA on cartas pra imprimir Mewtwo, Pokemon cards, Cool pokemon cards11 abril 2025
Pin by EWERTON DA on cartas pra imprimir Mewtwo, Pokemon cards, Cool pokemon cards11 abril 2025 -
 Pin de Tainá Pavanello em Bolo niver11 abril 2025
Pin de Tainá Pavanello em Bolo niver11 abril 2025 -
 780 KNB ideas in 2023 kuroko no basket, kuroko, kuroko's basketball11 abril 2025
780 KNB ideas in 2023 kuroko no basket, kuroko, kuroko's basketball11 abril 2025 -
 Alliterative Anime Character Click Quiz11 abril 2025
Alliterative Anime Character Click Quiz11 abril 2025 -
 Star Wars™ Galaxy of Heroes - Free Mobile Game - EA Official Site11 abril 2025
Star Wars™ Galaxy of Heroes - Free Mobile Game - EA Official Site11 abril 2025 -
 DUB Cars USA APK 5.7.6 for Android – Download DUB Cars USA APK Latest Version from11 abril 2025
DUB Cars USA APK 5.7.6 for Android – Download DUB Cars USA APK Latest Version from11 abril 2025 -
error 1001 on roblox pics|TikTok Search11 abril 2025