Treatment outcomes following continuous miglustat therapy in
Por um escritor misterioso
Last updated 02 abril 2025
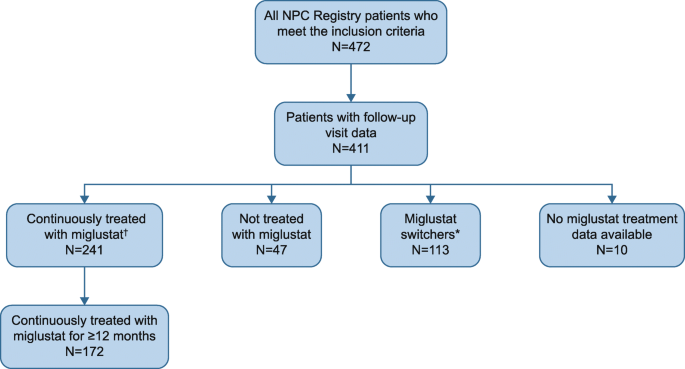
Background Niemann-Pick disease Type C (NP-C) is a rare, progressive neurodegenerative disorder characterized by progressive neurodegeneration and premature death. We report data at closure of the NPC Registry that describes the natural history, disease course and treatment experience of NP-C patients in a real-world setting. Methods The NPC Registry was a prospective observational cohort study that ran between September 2009 and October 2017. Patients with a confirmed diagnosis of NP-C were enrolled regardless of treatment status. All patients underwent clinical assessments and medical care as determined by their physicians; data were collected through a secure internet-based portal. Results At closure on October 19, 2017, 472 patients from 22 countries were enrolled in the NPC Registry. Mean (standard deviation) age at enrollment was 21.2 (15.0) years, and 51.9% of patients were male. First neurological symptom onset occurred during the early-infantile (< 2 years), late-infantile (2 to < 6 years), juvenile (6 to < 15 years), or adolescent/adult (≥ 15 years) periods in 13.5, 25.6, 31.8, and 29.1% of cases, respectively. The most frequent neurological manifestations prior to enrollment included ataxia (67.9%), vertical supranuclear gaze palsy (67.4%), dysarthria (64.7%), cognitive impairment (62.7%), dysphagia (49.1%), and dystonia (40.2%). During infancy, splenomegaly and hepatomegaly were frequent (n = 199/398 [50%] and n = 147/397 [37.0%], respectively) and persisted in most affected patients. Of the 472 enrolled patients, 241 were continuously treated with miglustat during the NPC Registry observation period, of whom 172 of these 241 patients were treated continuously for ≥12 months. A composite disability score that assesses impairment of ambulation, manipulation, language, and swallowing was highest in the early-infantile population and lowest in the adolescent/adult population. Among the continuous miglustat therapy population, 70.5% of patients had improved or had stable disease (at least 3 of the 4 domains having a decreased or unchanged score between enrollment and last follow-up). The NPC Registry did not identify any new safety signals associated with miglustat therapy. Conclusions The profiles of clinical manifestations in the final NPC Registry dataset agreed with previous clinical descriptions. Miglustat therapy was associated with a stabilization of neurological manifestations in most patients. The safety and tolerability of miglustat therapy was consistent with previous reports.
Frontiers Therapeutic Strategies For Tay-Sachs Disease

EP2796457A1 - Genz 112638 for treating Gaucher or Fabry disease in

Therapeutic advantages of combined gene/cell therapy strategies in
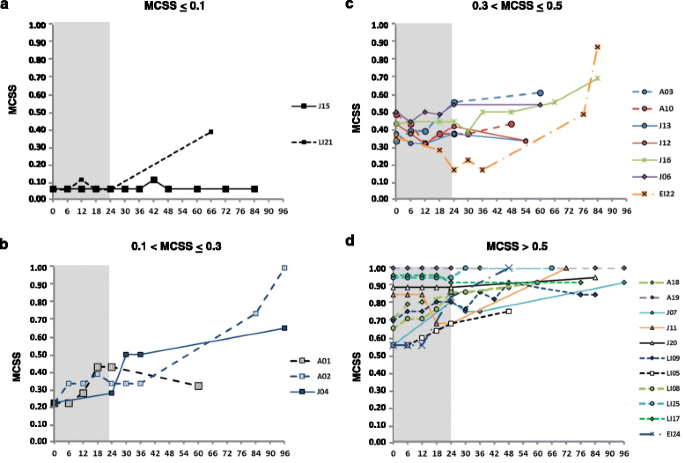
Long term follow-up to evaluate the efficacy of miglustat
Opfolda: Package Insert

Randomized, controlled trial of miglustat in Gaucher's disease

Miglustat in late-onset Tay-Sachs disease: a 12-month, randomized

Recommendations for oral treatment for adult patients with type 1

PDF] Miglustat for treatment of Niemann-Pick C disease: a

Full article: New therapies in the management of Niemann-Pick type

Treatment outcomes following continuous miglustat therapy in

CNS-accessible Inhibitor of Glucosylceramide Synthase for
Recomendado para você
-
 A new regulatory mechanism of STARD1 in Niemann-Pick disease type C (NPC), discovered02 abril 2025
A new regulatory mechanism of STARD1 in Niemann-Pick disease type C (NPC), discovered02 abril 2025 -
 NNPDF on X: October is Global Niemann-Pick Disease Awareness Month! For more information on Niemann-Pick Disease or to make a donation to NNPDF go to #niemannpick #ASMD #NPC #raredisease #NNPDF #NiemannPickC02 abril 2025
NNPDF on X: October is Global Niemann-Pick Disease Awareness Month! For more information on Niemann-Pick Disease or to make a donation to NNPDF go to #niemannpick #ASMD #NPC #raredisease #NNPDF #NiemannPickC02 abril 2025 -
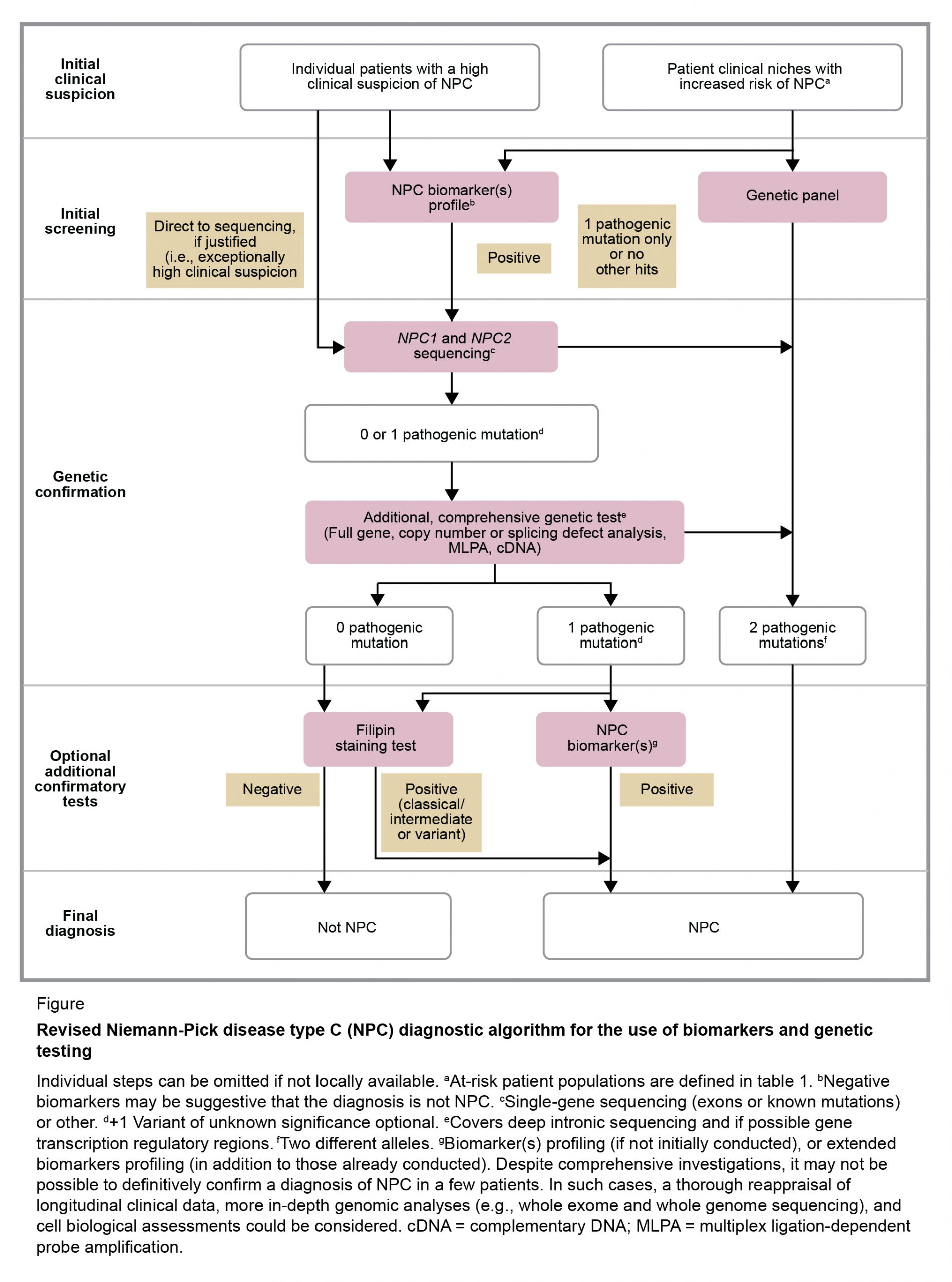 Niemann–Pick Disease Type C02 abril 2025
Niemann–Pick Disease Type C02 abril 2025 -
National Niemann-Pick Disease Foundation, Inc. - October is Global02 abril 2025
-
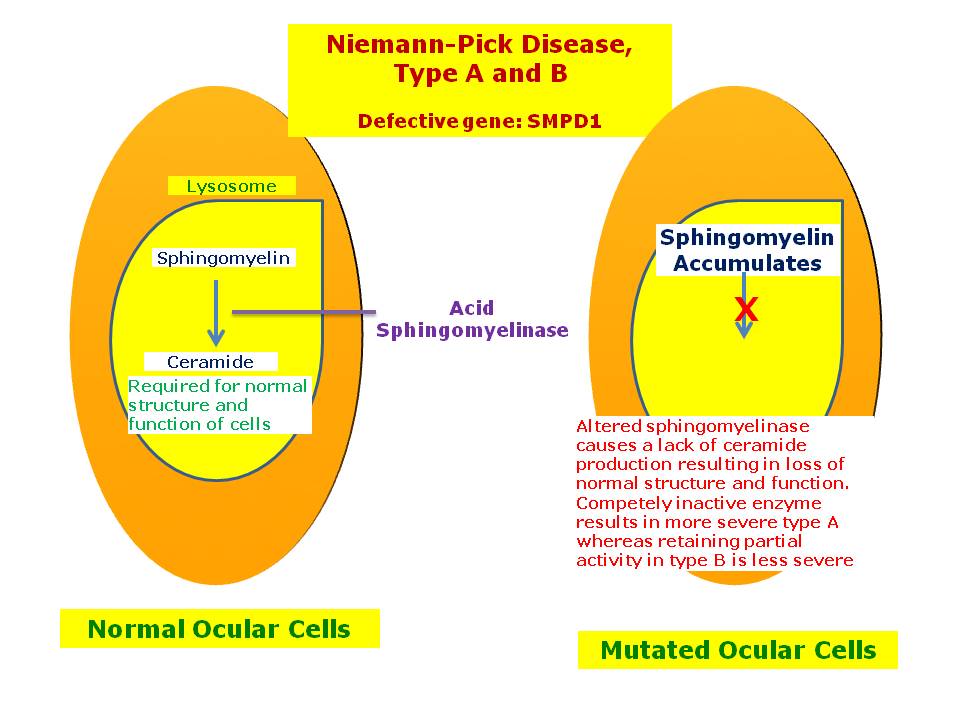 Niemann-Pick Disease, Types A and B02 abril 2025
Niemann-Pick Disease, Types A and B02 abril 2025 -
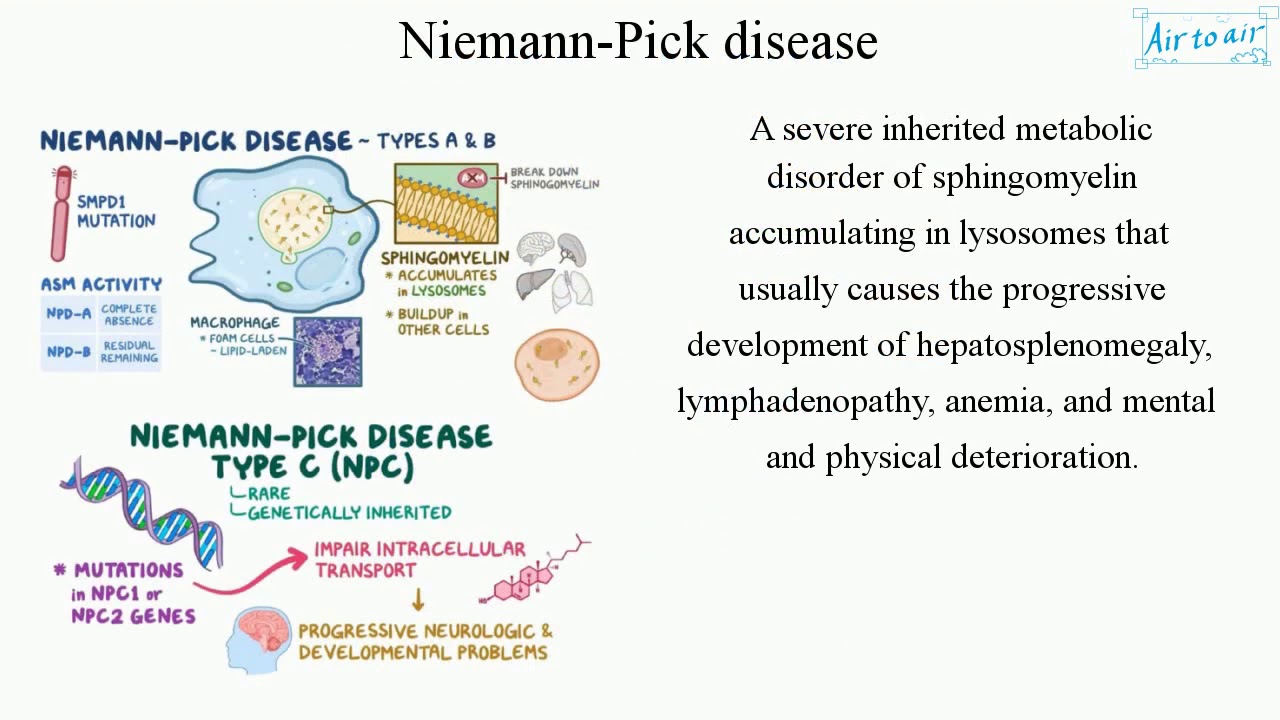 Niemann Pick disease02 abril 2025
Niemann Pick disease02 abril 2025 -
Niemann–Pick disease02 abril 2025
-
 The Importance of Newborn Screening for Niemann-Pick Disease – NNPDF02 abril 2025
The Importance of Newborn Screening for Niemann-Pick Disease – NNPDF02 abril 2025 -
National Niemann-Pick Disease Foundation, Inc. - October is Global02 abril 2025
-
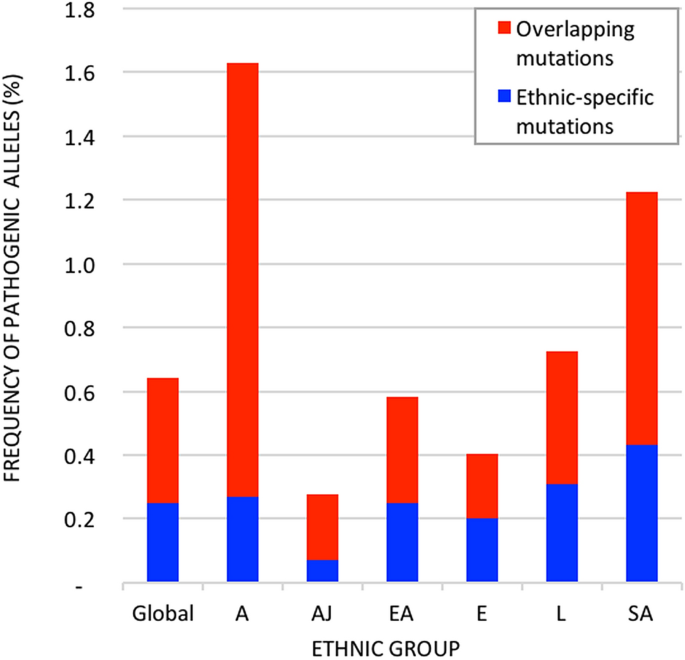 Signatures of natural selection and ethnic-specific prevalence of02 abril 2025
Signatures of natural selection and ethnic-specific prevalence of02 abril 2025
você pode gostar
-
 31 - LIVE! Gen 1 Shiny Starter Bulbasaur in Red after Only 464 SR02 abril 2025
31 - LIVE! Gen 1 Shiny Starter Bulbasaur in Red after Only 464 SR02 abril 2025 -
 17 Kid-Themed Hotel Rooms That Will Delight the Whole Family (2023) - FamilyVacationist02 abril 2025
17 Kid-Themed Hotel Rooms That Will Delight the Whole Family (2023) - FamilyVacationist02 abril 2025 -
 JobDoughBoi on X: 2017 sonic.exe canon / X02 abril 2025
JobDoughBoi on X: 2017 sonic.exe canon / X02 abril 2025 -
 Instagram Knb Kuroko, Kuroko no basket, Anime02 abril 2025
Instagram Knb Kuroko, Kuroko no basket, Anime02 abril 2025 -
 38 Picture id's for bloxburg ideas bloxburg decal codes, custom decals, room decals02 abril 2025
38 Picture id's for bloxburg ideas bloxburg decal codes, custom decals, room decals02 abril 2025 -
 Governor of Poker 3 Free - Online Game - Play for Free02 abril 2025
Governor of Poker 3 Free - Online Game - Play for Free02 abril 2025 -
 Krystal02 abril 2025
Krystal02 abril 2025 -
 WDN - World Dubbing News on X: 🥷 Novos dubladores juntam-se ao02 abril 2025
WDN - World Dubbing News on X: 🥷 Novos dubladores juntam-se ao02 abril 2025 -
 Pokemon Shodo - Kit Mew, Mewtwo e Charizard - Bandai em Promoção02 abril 2025
Pokemon Shodo - Kit Mew, Mewtwo e Charizard - Bandai em Promoção02 abril 2025 -
 Mortgage rates are falling. Should you refinance your home now? - CBS News02 abril 2025
Mortgage rates are falling. Should you refinance your home now? - CBS News02 abril 2025

