Early Safety Assessment - Drug Discovery and Development Based on
Por um escritor misterioso
Last updated 14 abril 2025

The drug candidate faces numerous efficacy and safety hurdles before moving forward to clinical testing. Here at the UPDDI we recognize the need for early identification of potential human toxicity and pharmacokinetic issues by creating a unique human liver microphysiological systems platform for drug testing before implementing preclinical animal testing.
Certara, Blog
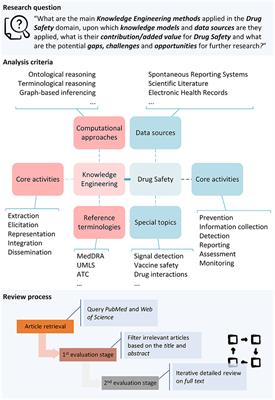
Frontiers Computational Advances in Drug Safety: Systematic and

Target safety assessments – identifying risk early

Organ-on-a-Chip: A New Paradigm for Drug Development: Trends in

The Biopharmaceutics Risk Assessment Roadmap for Optimizing

Drug Discovery and Development: An Overview - ScienceDirect

Early Safety Assessment

Toxicology Strategies for Drug Discovery: Present and Future

Drug development - Wikipedia

Why 90% of clinical drug development fails and how to improve it

Early Safety Assessment - Drug Discovery and Development Based on

Regulatory Readiness Level: a Tool to Enhance Early Regulatory
Recomendado para você
-
brain test 196|TikTok Search14 abril 2025
-
 BRAIN TEST NÍVEL 411 EM PORTUGUÊS14 abril 2025
BRAIN TEST NÍVEL 411 EM PORTUGUÊS14 abril 2025 -
 Easy Game - Brain Test Level 411-420 Walkthrough Solution (iOS14 abril 2025
Easy Game - Brain Test Level 411-420 Walkthrough Solution (iOS14 abril 2025 -
 Brain Test Tricky puzzles level (411-420)14 abril 2025
Brain Test Tricky puzzles level (411-420)14 abril 2025 -
 The 411 on A1C: Normal A1C levels and 15 ways to lower high A1C14 abril 2025
The 411 on A1C: Normal A1C levels and 15 ways to lower high A1C14 abril 2025 -
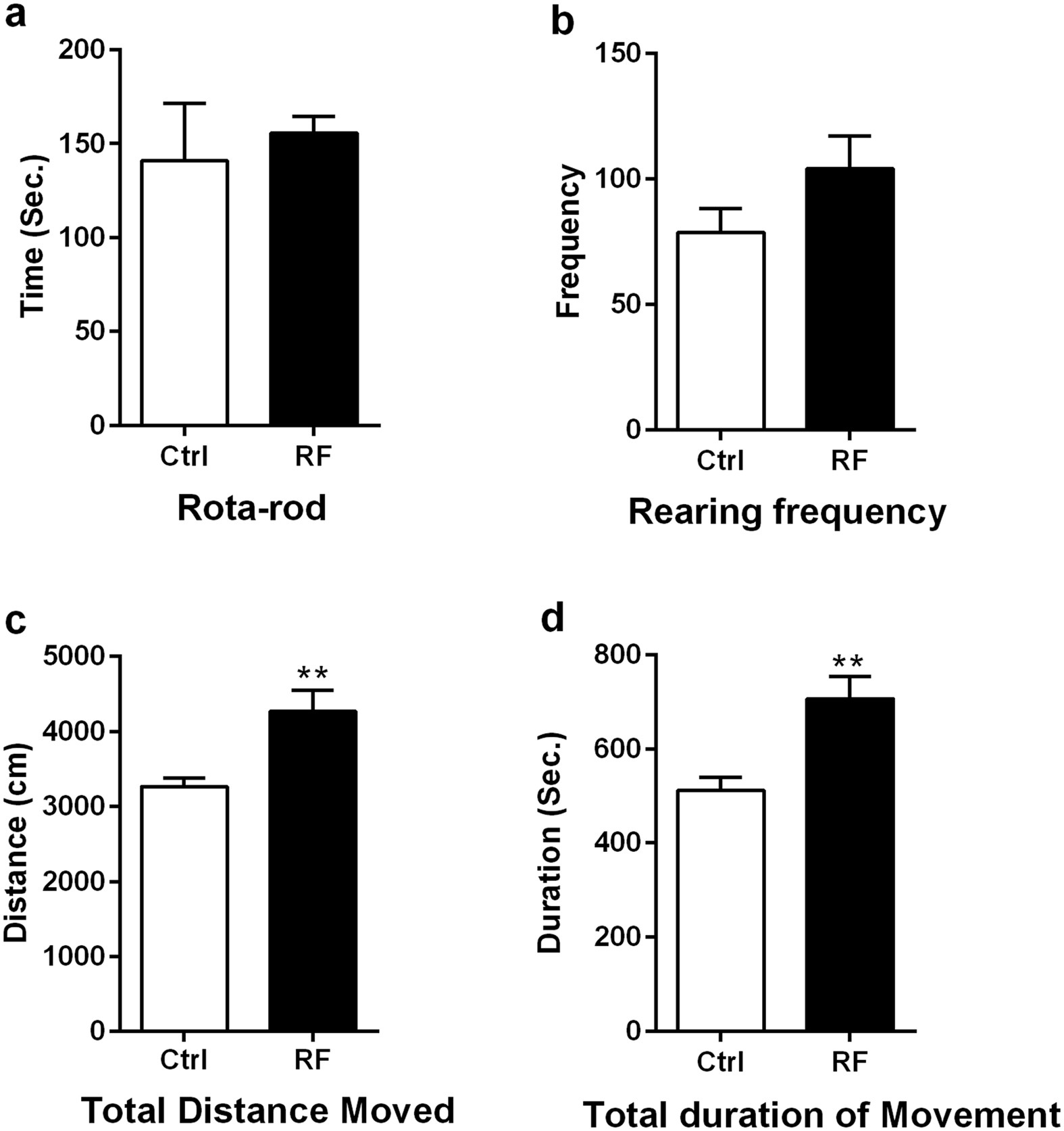 Long-term exposure to 835 MHz RF-EMF induces hyperactivity14 abril 2025
Long-term exposure to 835 MHz RF-EMF induces hyperactivity14 abril 2025 -
 208 And 482 In 1 Game Card, Super Combo Game14 abril 2025
208 And 482 In 1 Game Card, Super Combo Game14 abril 2025 -
 Cachexia - Wikipedia14 abril 2025
Cachexia - Wikipedia14 abril 2025 -
 anoosha syed14 abril 2025
anoosha syed14 abril 2025 -
 Wernicke's & Broca's aphasia Brain & Language LING 411/412/48914 abril 2025
Wernicke's & Broca's aphasia Brain & Language LING 411/412/48914 abril 2025
você pode gostar
-
 Independiente, 10° en el ranking de clubes de Conmebol14 abril 2025
Independiente, 10° en el ranking de clubes de Conmebol14 abril 2025 -
 futbol nacional uruguay!!! Real madrid, Barcelona futbol club, Madrid14 abril 2025
futbol nacional uruguay!!! Real madrid, Barcelona futbol club, Madrid14 abril 2025 -
 Download TSM APKs for Android - APKMirror14 abril 2025
Download TSM APKs for Android - APKMirror14 abril 2025 -
 Bunzo Bunny Plush Toy14 abril 2025
Bunzo Bunny Plush Toy14 abril 2025 -
BORUTO: NARUTO NEXT GENERATIONS Breakthrough - Watch on Crunchyroll14 abril 2025
-
 Skolas Kell of the Eliksni, Wiki14 abril 2025
Skolas Kell of the Eliksni, Wiki14 abril 2025 -
The idea behind this R36 Nissan Skyline GT-R concept was to14 abril 2025
-
 Abandoned Shrine of the Arch Terrarian: Boss Battle Arena Terrarium base, Terraria house design, Terraria house ideas14 abril 2025
Abandoned Shrine of the Arch Terrarian: Boss Battle Arena Terrarium base, Terraria house design, Terraria house ideas14 abril 2025 -
 One Piece Film: RED Spoiler Talk (FULL SUMMARY) – The Library of Ohara14 abril 2025
One Piece Film: RED Spoiler Talk (FULL SUMMARY) – The Library of Ohara14 abril 2025 -
 Brasil Trivia and Quizzes - TriviaCreator14 abril 2025
Brasil Trivia and Quizzes - TriviaCreator14 abril 2025

