Top 4 eConsent Questions in Clinical Research: Forms & More
Por um escritor misterioso
Last updated 01 abril 2025
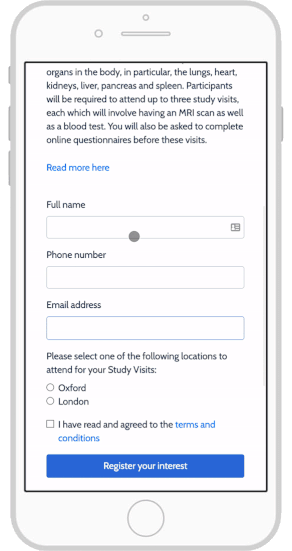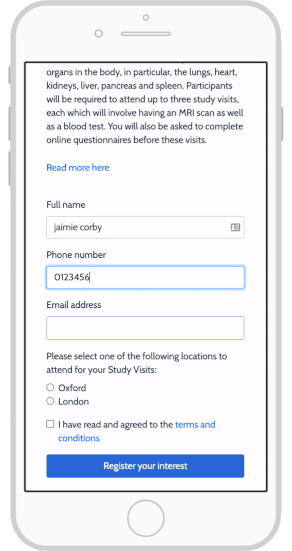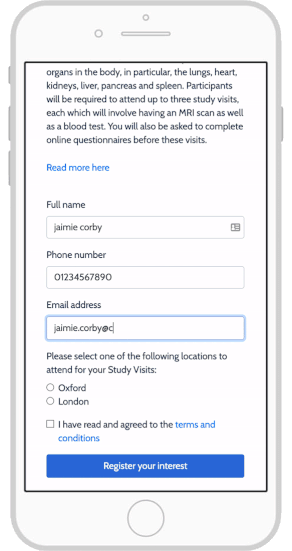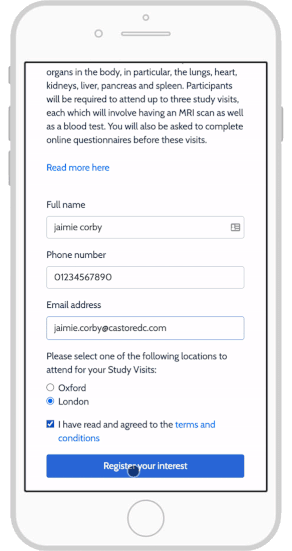

eConsent: Sites and Sponsors Tout Benefits, Confront Obstacles
eConsent In Healthcare Market Demand and Future Scope Analysis
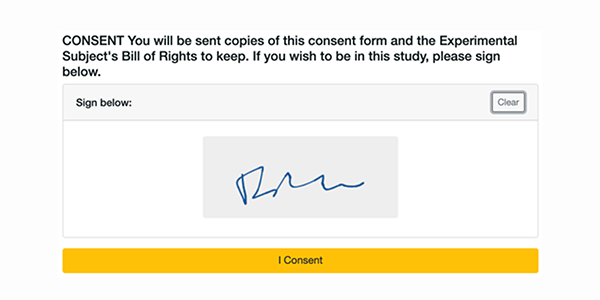
Using e-Consent Forms in Your Clinical Research
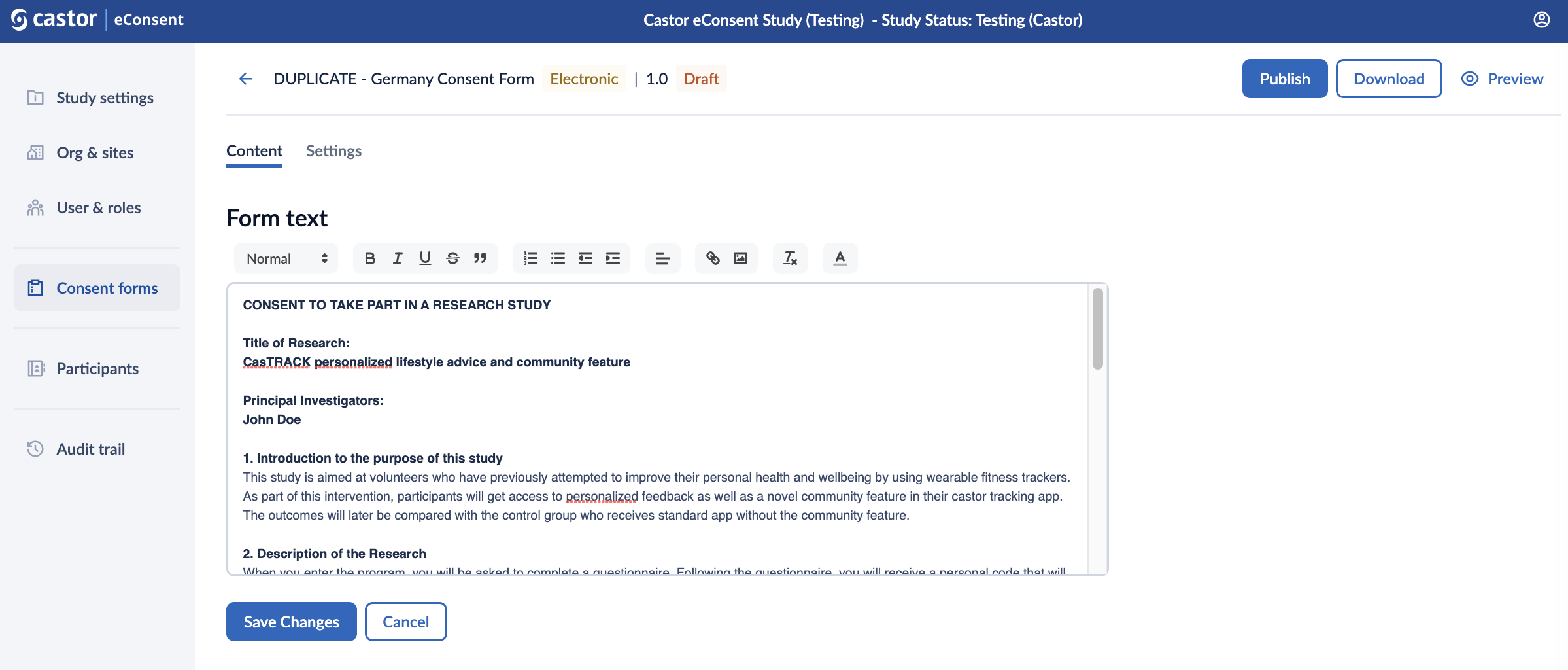
Adding and editing a consent form in eConsent - Castor

7 ways eConsent can help health researchers achieve better

Electronic Consent Form Template - Fill Online, Printable
How to Avoid the Top 5 Clinical Trial FDA Inspection Failures

eConsent—The First Step to Enable Clinical Trial Access to Anyone

Paving the way to a more effective informed consent process

E consent for research: Considerations in Implementation and IRB
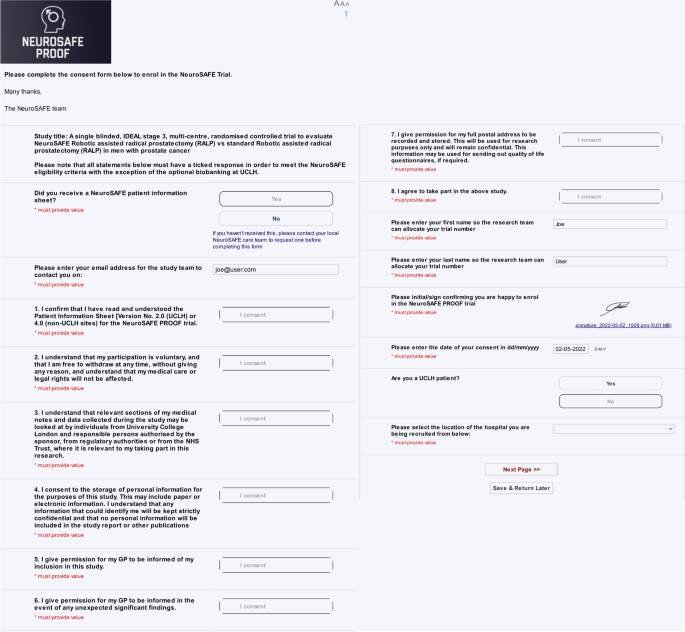
E-Consent—a guide to maintain recruitment in clinical trials

Awareness and Collaboration Across Stakeholder Groups Important
Recomendado para você
-
 patrizia Love heart gif, Online badge maker, Heart wallpaper01 abril 2025
patrizia Love heart gif, Online badge maker, Heart wallpaper01 abril 2025 -
 GitHub - paulfitz/makesweet: Put pictures into animations from the command line.01 abril 2025
GitHub - paulfitz/makesweet: Put pictures into animations from the command line.01 abril 2025 -
 Heart Locket GIF01 abril 2025
Heart Locket GIF01 abril 2025 -
 Heart Animation by Qianxu Zeng on Dribbble01 abril 2025
Heart Animation by Qianxu Zeng on Dribbble01 abril 2025 -
 Online Banner Maker - Badge with Centered Text by Placeit on Dribbble01 abril 2025
Online Banner Maker - Badge with Centered Text by Placeit on Dribbble01 abril 2025 -
 Welcome: Red Heart01 abril 2025
Welcome: Red Heart01 abril 2025 -
 People's Daily online exhorts China to 'stab at the heart of the snake' in response to U.S. tariffs, Top News01 abril 2025
People's Daily online exhorts China to 'stab at the heart of the snake' in response to U.S. tariffs, Top News01 abril 2025 -
 Virtual and Online Love Letter - Virtual Gifts at01 abril 2025
Virtual and Online Love Letter - Virtual Gifts at01 abril 2025 -
 The Changing Online Language of Hearts - The New York Times01 abril 2025
The Changing Online Language of Hearts - The New York Times01 abril 2025 -
Unchain My heART connects kids with community – The Durango Herald01 abril 2025
você pode gostar
-
 66 Animal Combinations for Little Alchemy 201 abril 2025
66 Animal Combinations for Little Alchemy 201 abril 2025 -
 Miri Mikawa Sugar Apple Fairy Tale Collector's Edition 1 Kadokawa01 abril 2025
Miri Mikawa Sugar Apple Fairy Tale Collector's Edition 1 Kadokawa01 abril 2025 -
 Soy Kage01 abril 2025
Soy Kage01 abril 2025 -
 Yōsuke Yoneya, World Trigger Wiki01 abril 2025
Yōsuke Yoneya, World Trigger Wiki01 abril 2025 -
Boneco Pokemon Miniatura Ash Greninja Lendarios Tomy | Brinquedo Tomy Nunca Usado 45726676 | enjoei01 abril 2025
-
 HELP! i cant figure out how to do i start this puzzle. ive tried01 abril 2025
HELP! i cant figure out how to do i start this puzzle. ive tried01 abril 2025 -
 Kanojo, Okarishimasu' Receives Second Season01 abril 2025
Kanojo, Okarishimasu' Receives Second Season01 abril 2025 -
 Uncharted 4 on PC at 100+ fps is AMAZING01 abril 2025
Uncharted 4 on PC at 100+ fps is AMAZING01 abril 2025 -
 Como desenhar rosto do vegeta - Como desenhar01 abril 2025
Como desenhar rosto do vegeta - Como desenhar01 abril 2025 -
 Haikyu Kei Tsukishima 4K HD Anime Wallpapers, HD Wallpapers01 abril 2025
Haikyu Kei Tsukishima 4K HD Anime Wallpapers, HD Wallpapers01 abril 2025
