FDA's Fast-Track for Rexulti Raises Concerns
Por um escritor misterioso
Last updated 03 abril 2025

CMS efforts to reduce use of unnecessary antipsychotics in nursing homes may conflict with marketing efforts for the drug.
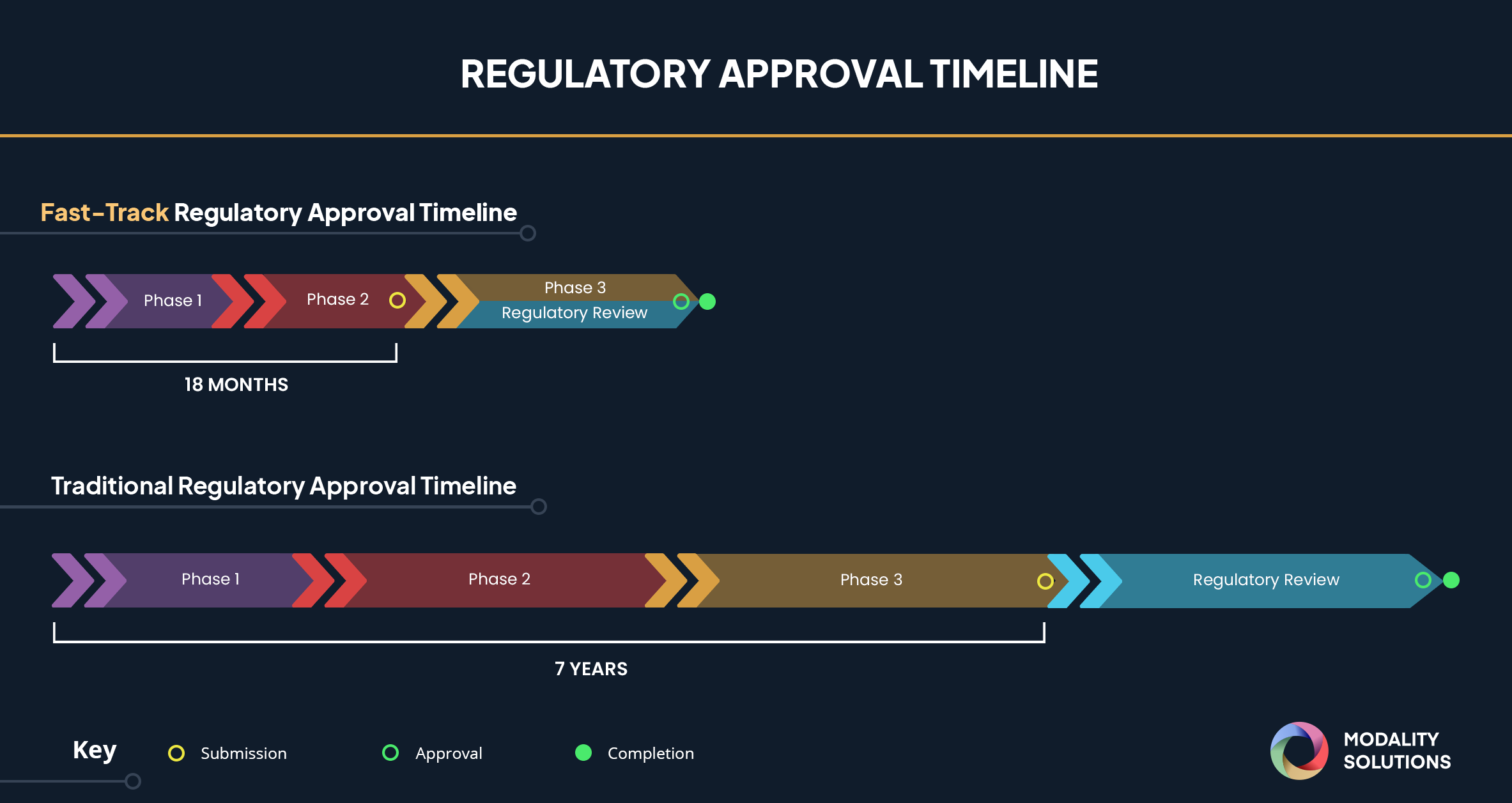
FDA's Fast Track Approval Coronavirus Treatment Acceleration Program

Not Everyone Agreed With FDA Approval of Antipsychotic Rexulti for Agitation - Mad In America

FDA Fast Track Designation for Ad-RTS-hIL-12 plus Veledimex for Recurrent Glioblastoma

Listen to New FDA Approvals podcast
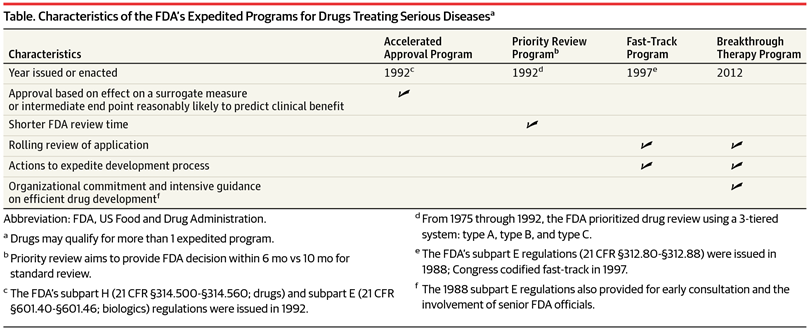
The Science Of A Biotech Valuation: How To Interpret The Value Of FDA Expedited Programs (NASDAQ:IBB)

FDA fast-tracks drug for dementia agitation that increases risk of death 4 times - Study Finds

Amgen Triumphs in $27.8 Billion Horizon Buyout Battle

Rare Disease Clinical Trials: Strategies Learned from Duchenne Muscular Dystrophy
FDA fast-tracks drug for dementia agitation that increases risk of death 4 times - Study Finds
Recomendado para você
-
 REXULTI® (brexpiprazole) Patient Information Website03 abril 2025
REXULTI® (brexpiprazole) Patient Information Website03 abril 2025 -
 Everything you NEED to Know about Rexulti (Brexpiprazole)03 abril 2025
Everything you NEED to Know about Rexulti (Brexpiprazole)03 abril 2025 -
What you need to know about Rexulti (Brexpiprazole)03 abril 2025
-
 REXULTI (brexpiprazole) Tablet03 abril 2025
REXULTI (brexpiprazole) Tablet03 abril 2025 -
 FDA Approves Rexulti For Agitation Associated With Dementia Due To Alzheimer's03 abril 2025
FDA Approves Rexulti For Agitation Associated With Dementia Due To Alzheimer's03 abril 2025 -
 Introducing REXULTI® (brexpiprazole): a new PBS-listed antipsychotic for schizophrenia03 abril 2025
Introducing REXULTI® (brexpiprazole): a new PBS-listed antipsychotic for schizophrenia03 abril 2025 -
REXULTI® (brexpiprazole), MDD03 abril 2025
-
 Rexulti 3mg Tablet03 abril 2025
Rexulti 3mg Tablet03 abril 2025 -
 Rexulti 1mg Lundbeck 30 Comprimidos - Drogaria Sao Paulo03 abril 2025
Rexulti 1mg Lundbeck 30 Comprimidos - Drogaria Sao Paulo03 abril 2025 -
 Rexulti For Depression: Benefits, Side Effects & Precautions03 abril 2025
Rexulti For Depression: Benefits, Side Effects & Precautions03 abril 2025
você pode gostar
-
 Cat meme / Reaction pic / Drawing style : r/HelpMeFind03 abril 2025
Cat meme / Reaction pic / Drawing style : r/HelpMeFind03 abril 2025 -
 NRL 2023, Brisbane Broncos, Penrith Panthers, round 12 preview, official team lists, updates, injuries03 abril 2025
NRL 2023, Brisbane Broncos, Penrith Panthers, round 12 preview, official team lists, updates, injuries03 abril 2025 -
 5 Fatos que você não sabe sobre o Super Saiyajin 303 abril 2025
5 Fatos que você não sabe sobre o Super Saiyajin 303 abril 2025 -
 Gato kawai anime, videojuegos03 abril 2025
Gato kawai anime, videojuegos03 abril 2025 -
 Resident Evil Code Veronica - Fan Arte Poster by vinycalheiros on03 abril 2025
Resident Evil Code Veronica - Fan Arte Poster by vinycalheiros on03 abril 2025 -
 Deck Case Shining Gardevoir Pokémon Card Game - Meccha Japan03 abril 2025
Deck Case Shining Gardevoir Pokémon Card Game - Meccha Japan03 abril 2025 -
 Probably the most scathing review of the Marvels yet : r/MauLer03 abril 2025
Probably the most scathing review of the Marvels yet : r/MauLer03 abril 2025 -
 Max Payne 2 - photoshoot (2003) : r/gaming03 abril 2025
Max Payne 2 - photoshoot (2003) : r/gaming03 abril 2025 -
 Second Half of Attack on Titan Final Season Part 3 Airs Fall 202303 abril 2025
Second Half of Attack on Titan Final Season Part 3 Airs Fall 202303 abril 2025 -
 How to Install Minecraft 1.20 & Minecraft Update News 22w42a03 abril 2025
How to Install Minecraft 1.20 & Minecraft Update News 22w42a03 abril 2025
