Contributing to Evidence-Based Regulatory Decisions: A Comparison
Por um escritor misterioso
Last updated 04 abril 2025

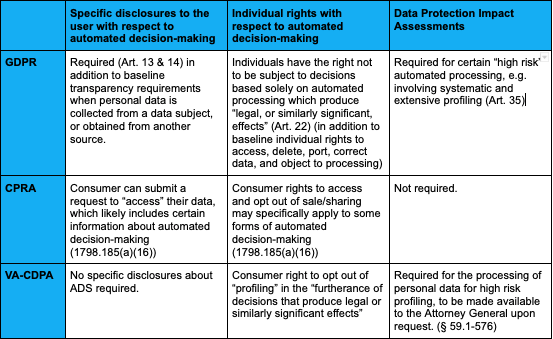
Automated-Decision Making Systems: Considerations for State Policymakers
:max_bytes(150000):strip_icc()/what-difference-between-state-and-federally-chartered-credit-union_final-6d23ae652fc34b3d96a7ce26e31b4543.png)
State vs. Federally Chartered Credit Unions: What's the Difference?

Sigma Marketplace. ⁰Johns Hopkins Evidence-Based Practice for Nurses, Fourth Edition - Updated Content

Same Science, Different Policies: Regulating Genetically Modified Foods in the U.S. and Europe - Science in the News

Real-world evidence (RWE) in regulatory decision making: key use cases.

Necessity of strengthening the current clinical regulatory for companion diagnostics: An institutional comparison of the FDA, EMA, and MFDS: Molecular Therapy - Methods & Clinical Development

Abortion Worldwide 2017: Uneven Progress and Unequal Access
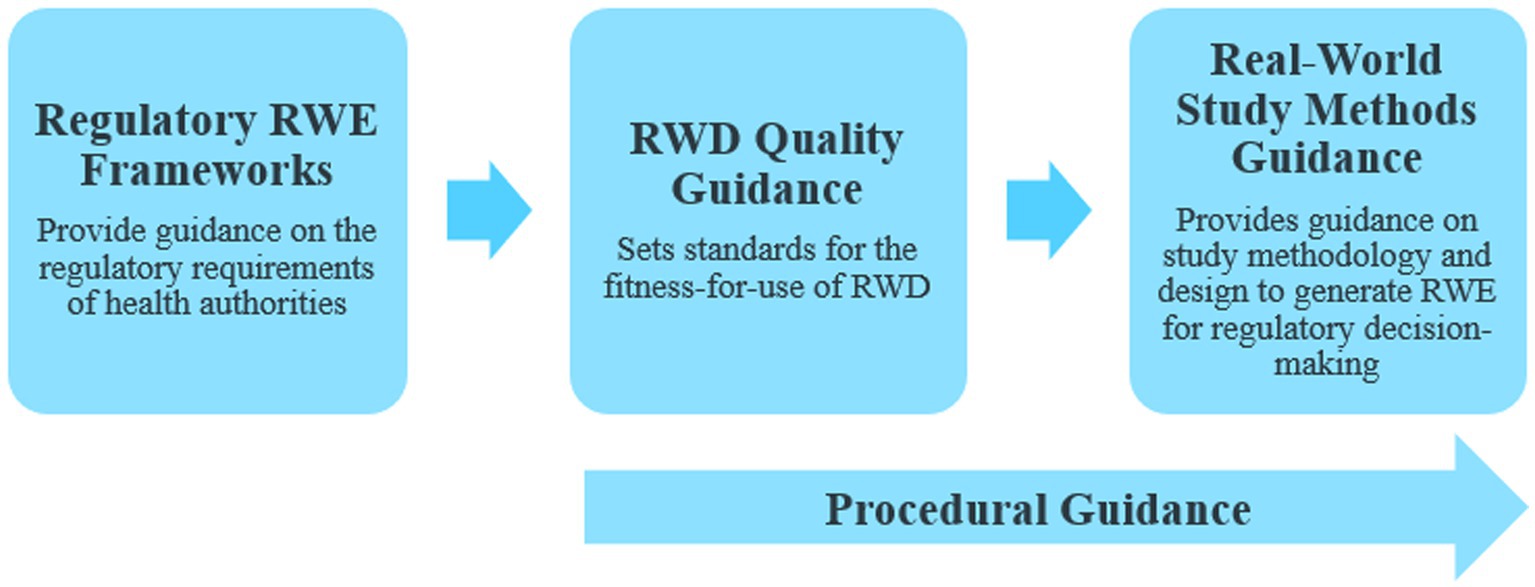
Frontiers Real-world evidence for regulatory decision-making: updated guidance from around the world

Evidence-Based Medicine Framework for Clinical Decision Making Adapted

The EU and U.S. diverge on AI regulation: A transatlantic comparison and steps to alignment
Recomendado para você
-
 Banished Words Listed By Year 1976 - 202204 abril 2025
Banished Words Listed By Year 1976 - 202204 abril 2025 -
 What Does I Forgor 💀 Mean?04 abril 2025
What Does I Forgor 💀 Mean?04 abril 2025 -
 The Enigmatic Science Fiction of Djuna04 abril 2025
The Enigmatic Science Fiction of Djuna04 abril 2025 -
Example entry on Urban Dictionary, including the head word (104 abril 2025
-
 Femlandia04 abril 2025
Femlandia04 abril 2025 -
 Role of ectopic olfactory receptors in glucose and lipid04 abril 2025
Role of ectopic olfactory receptors in glucose and lipid04 abril 2025 -
 Proceedings of the 2023 SIAM International Conference on Data04 abril 2025
Proceedings of the 2023 SIAM International Conference on Data04 abril 2025 -
 Regenerative Agriculture: Definition04 abril 2025
Regenerative Agriculture: Definition04 abril 2025 -
 The Second Tale The Old Man on the Island in: The Culture of Latin04 abril 2025
The Second Tale The Old Man on the Island in: The Culture of Latin04 abril 2025 -
 Emo, love and god: making sense of Urban Dictionary, a crowd04 abril 2025
Emo, love and god: making sense of Urban Dictionary, a crowd04 abril 2025
você pode gostar
-
 Animehouse — Mahou Shoujo Magical Destroyers Episode 4: R U04 abril 2025
Animehouse — Mahou Shoujo Magical Destroyers Episode 4: R U04 abril 2025 -
 Decidueye and inteleon are the best sniper partners IMO : r/PokemonUnite04 abril 2025
Decidueye and inteleon are the best sniper partners IMO : r/PokemonUnite04 abril 2025 -
 Jogo de quebra-cabeça para crianças berinjela de legumes peças de quebra- cabeça planilha de cores página de atividades04 abril 2025
Jogo de quebra-cabeça para crianças berinjela de legumes peças de quebra- cabeça planilha de cores página de atividades04 abril 2025 -
 Secretaria de Esportes e Recreação – Página: 100 – Prefeitura de04 abril 2025
Secretaria de Esportes e Recreação – Página: 100 – Prefeitura de04 abril 2025 -
 Midas Golden Touch - Play now with Crypto04 abril 2025
Midas Golden Touch - Play now with Crypto04 abril 2025 -
 Postagens DESTAQUES - Página 38 de 124 - MixMods04 abril 2025
Postagens DESTAQUES - Página 38 de 124 - MixMods04 abril 2025 -
 Tanjiro Adesivos gradientes Lâmina matadora de fantasma 3D Adesivos de ilusão de desenho animado Adesivos de carro que mudam de carro Adesivos de04 abril 2025
Tanjiro Adesivos gradientes Lâmina matadora de fantasma 3D Adesivos de ilusão de desenho animado Adesivos de carro que mudam de carro Adesivos de04 abril 2025 -
Shopee Brasil Ofertas incríveis. Melhores preços do mercado04 abril 2025
-
 Comics with Sans - Comic Studio04 abril 2025
Comics with Sans - Comic Studio04 abril 2025 -
 Guilty Crown Manga by animemangart on DeviantArt04 abril 2025
Guilty Crown Manga by animemangart on DeviantArt04 abril 2025
