ASpire ASDAS by Incuna Asia Pacific
Por um escritor misterioso
Last updated 04 abril 2025

The ASpire ASDAS application is an exciting new application designed designed by experts to help patients with Ankylosing Spondylitis (AS) manage and learn more about their condition
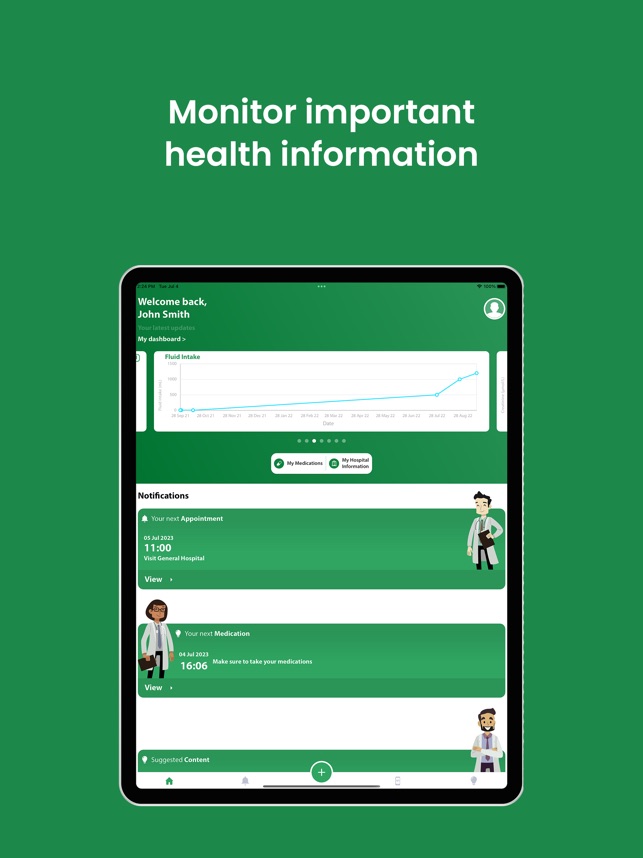
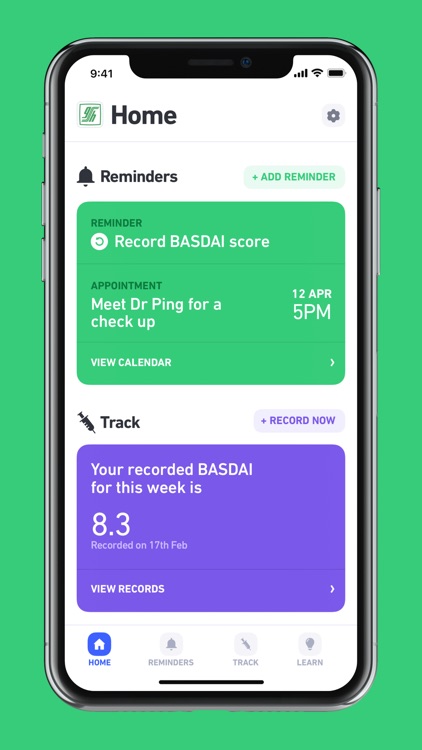
The ASpire ASDAS application is an exciting new application designed designed by experts to help patients with Ankylosing Spondylitis (AS) manage and learn more about their condition. Aspire App is multilingual and is available in English, Mandarin, Malay, Tamil and Bahasa language versions. ASpire provides you instant access to a comprehensive list of medical information, exercise videos and assessments to help you manage your AS better. ASpire includes both BASDAI and ASDAS questionnaires to assess the severity of the AS condition. Users can use these to monitor their condition over time and, if paired with a prescription, track any subsequent improvement. ASpire also includes a reminders feature to remind users when to record BASDAI/ASDAS scores, take their treatment, or attend an appointment at their hospital. Regular blood tests (CRP), if required can also be stored in this app.
The ASpire ASDAS application is an exciting new application designed designed by experts to help patients with Ankylosing Spondylitis (AS) manage and learn more about their condition. Aspire App is multilingual and is available in English, Mandarin, Malay, Tamil and Bahasa language versions. ASpire provides you instant access to a comprehensive list of medical information, exercise videos and assessments to help you manage your AS better. ASpire includes both BASDAI and ASDAS questionnaires to assess the severity of the AS condition. Users can use these to monitor their condition over time and, if paired with a prescription, track any subsequent improvement. ASpire also includes a reminders feature to remind users when to record BASDAI/ASDAS scores, take their treatment, or attend an appointment at their hospital. Regular blood tests (CRP), if required can also be stored in this app.

MySpA by Barts Health NHS Trust

Aspire Now Global - Crypto, Equity, Financial Modeling

Meetings – Pharmaleaders TV

Aspire Inc, Scientific Instruments

Aspire Inc. - Manufacturer from Bengaluru, India
iCommit Australia on the App Store

Aspire Inc, Scientific Instruments
ASpire ASDAS by Incuna Asia Pacific

QBE EO Guidelines.pdf

Aspire Inc. - Manufacturer from Bengaluru, India

Home - Center for Global Best Practices
Recomendado para você
-
 ASDAS states in patients stratified by baseline MRI/CRP status.04 abril 2025
ASDAS states in patients stratified by baseline MRI/CRP status.04 abril 2025 -
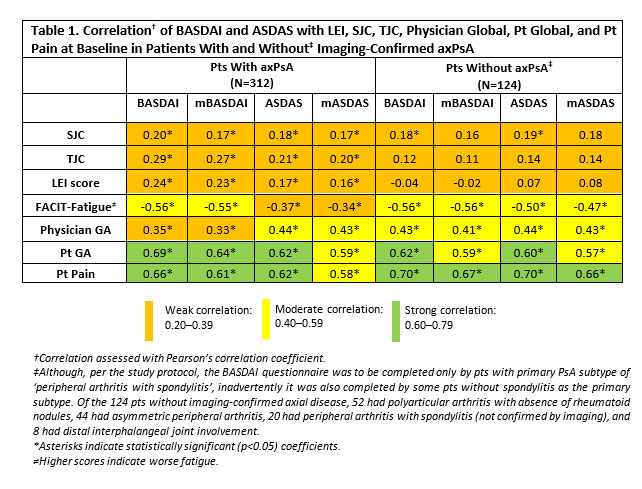 Performance of BASDAI vs. ASDAS in Evaluating Axial Involvement in Patients with PsA Treated with Guselkumab: Pooled Analysis of Two Phase 3 Studies - ACR Meeting Abstracts04 abril 2025
Performance of BASDAI vs. ASDAS in Evaluating Axial Involvement in Patients with PsA Treated with Guselkumab: Pooled Analysis of Two Phase 3 Studies - ACR Meeting Abstracts04 abril 2025 -
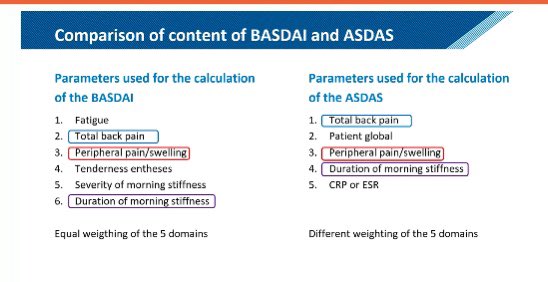 SMC Arthritis Forum/Dr.Hanady Manasfi on X: Comparison #BASDAI and #ASDAS #spondyloarthtopathy #EULAR2020 / X04 abril 2025
SMC Arthritis Forum/Dr.Hanady Manasfi on X: Comparison #BASDAI and #ASDAS #spondyloarthtopathy #EULAR2020 / X04 abril 2025 -
 Asda - Supermarkets Pesticide Ranking04 abril 2025
Asda - Supermarkets Pesticide Ranking04 abril 2025 -
Asdas Svg Png Icon Free Download (#77015)04 abril 2025
-
 Asdas Sticker - Asdas - Discover & Share GIFs04 abril 2025
Asdas Sticker - Asdas - Discover & Share GIFs04 abril 2025 -
Lissomeness Swelling - song and lyrics by asdas04 abril 2025
-
 About Us Asda Supplier04 abril 2025
About Us Asda Supplier04 abril 2025 -
 Asdas GIF - Asdas - Discover & Share GIFs04 abril 2025
Asdas GIF - Asdas - Discover & Share GIFs04 abril 2025 -
 asdas04 abril 2025
asdas04 abril 2025
você pode gostar
-
 Black Desert Mobile: Land of the Morning Light Update Unveiled04 abril 2025
Black Desert Mobile: Land of the Morning Light Update Unveiled04 abril 2025 -
 Western Movies to Watch in 202304 abril 2025
Western Movies to Watch in 202304 abril 2025 -
 Zahir (sometimes streams) on X: Best anime character tier list on this app 🥱 / X04 abril 2025
Zahir (sometimes streams) on X: Best anime character tier list on this app 🥱 / X04 abril 2025 -
 The Playoffs » Em primeiro jogo em Las Vegas, Raiders surpreendem04 abril 2025
The Playoffs » Em primeiro jogo em Las Vegas, Raiders surpreendem04 abril 2025 -
 Fnaf 1 freddy jumpscare transparent - lsabg04 abril 2025
Fnaf 1 freddy jumpscare transparent - lsabg04 abril 2025 -
stitch with @2pfrog @The one StrawHat @ITSDZL @BUGGY#1FAN #doffy😁 #o04 abril 2025
-
 The Best Anime Like KonoSuba04 abril 2025
The Best Anime Like KonoSuba04 abril 2025 -
 Flamengo fica no empate com Fluminense e afasta sonho do título04 abril 2025
Flamengo fica no empate com Fluminense e afasta sonho do título04 abril 2025 -
 Minecraft: Nintendo Switch Edition : Mojang, 4J Studios : Free04 abril 2025
Minecraft: Nintendo Switch Edition : Mojang, 4J Studios : Free04 abril 2025 -
 Dust Hill (underground) Map – RPG Insanity04 abril 2025
Dust Hill (underground) Map – RPG Insanity04 abril 2025

