Ethide Laboratories - USP 88 In-Vivo Cytotoxicity Testing
Por um escritor misterioso
Last updated 29 março 2025

Learn what USP 88 cytotoxicity tests are available and which ones you will need to meet the regulatory requirements for your medical devices.

New Insights into the Mechanism of Action of the Cyclopalladated Complex (CP2) in Leishmania: Calcium Dysregulation, Mitochondrial Dysfunction, and Cell Death
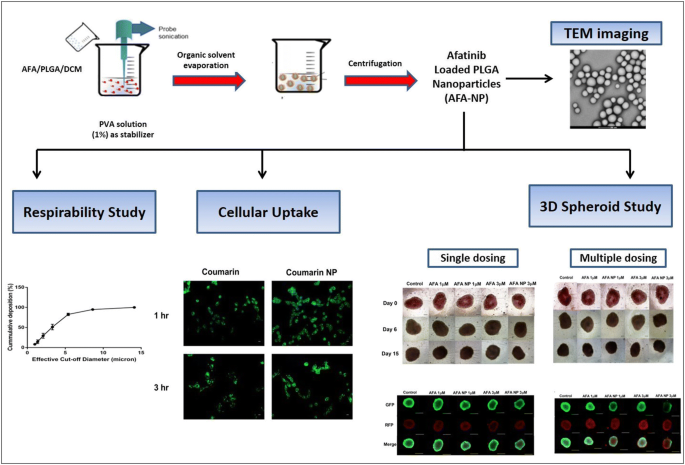
Afatinib-loaded inhalable PLGA nanoparticles for localized therapy of non-small cell lung cancer (NSCLC)—development and in-vitro efficacy

In Vivo Selectivity and Localization of Reactive Oxygen Species (ROS) Induction by Osmium Anticancer Complexes That Circumvent Platinum Resistance

EP2897610B1 - Pkc delta inhibitors for use as therapeutics - Google Patents
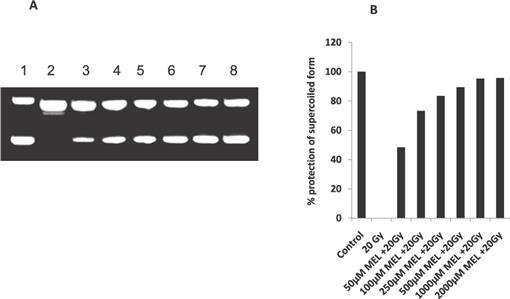
Sesamol as a Potential Radioprotective Agent: In Vitro Studies
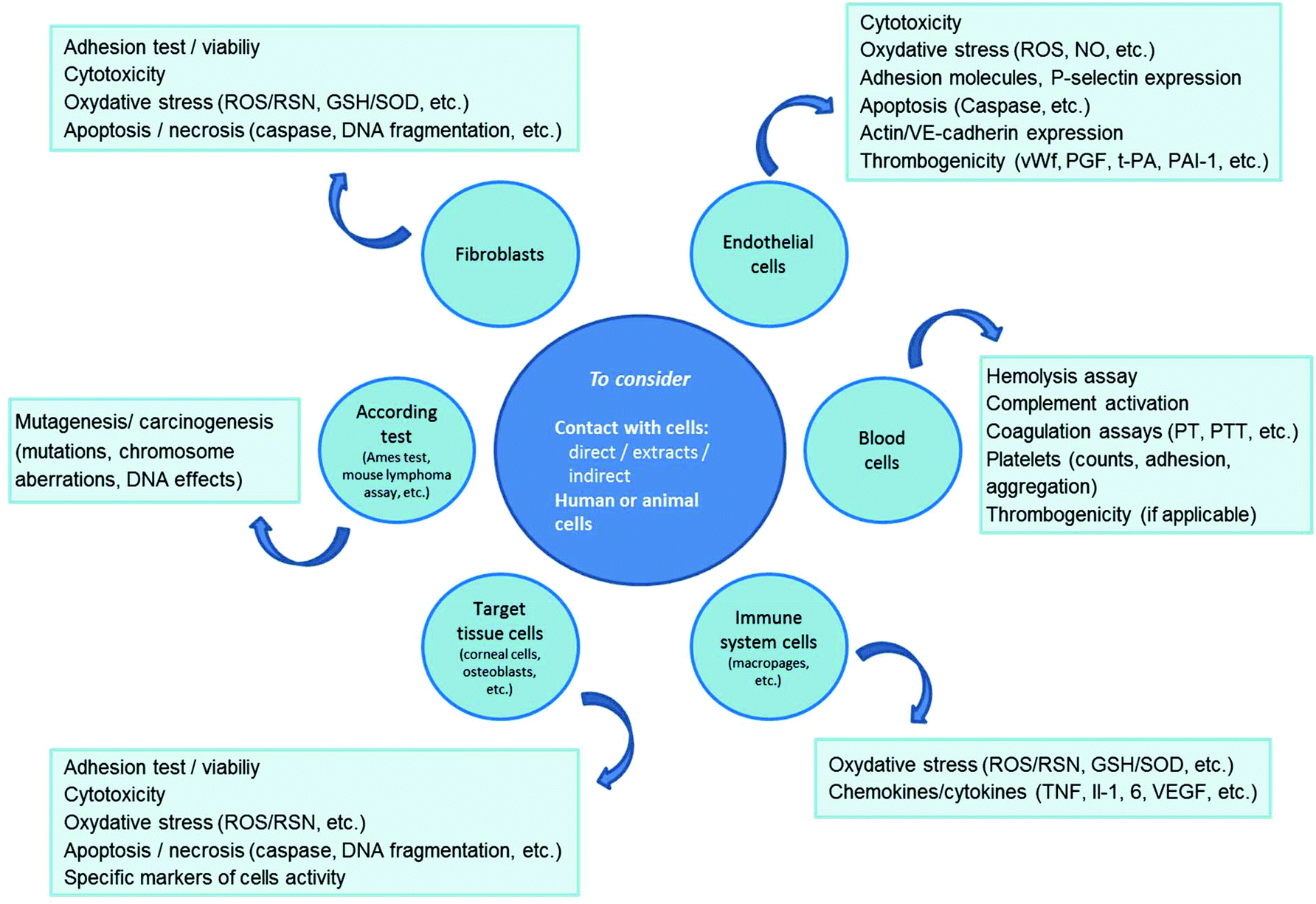
Biocompatibility of polymer-based biomaterials and medical devices – regulations, in vitro screening and risk-management - Biomaterials Science (RSC Publishing) DOI:10.1039/C8BM00518D
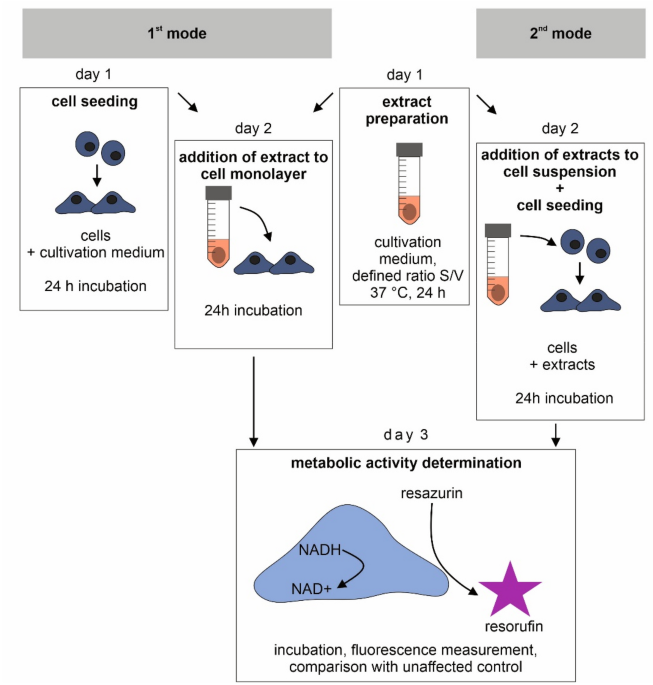
Test conditions can significantly affect the results of in vitro cytotoxicity testing of degradable metallic biomaterials
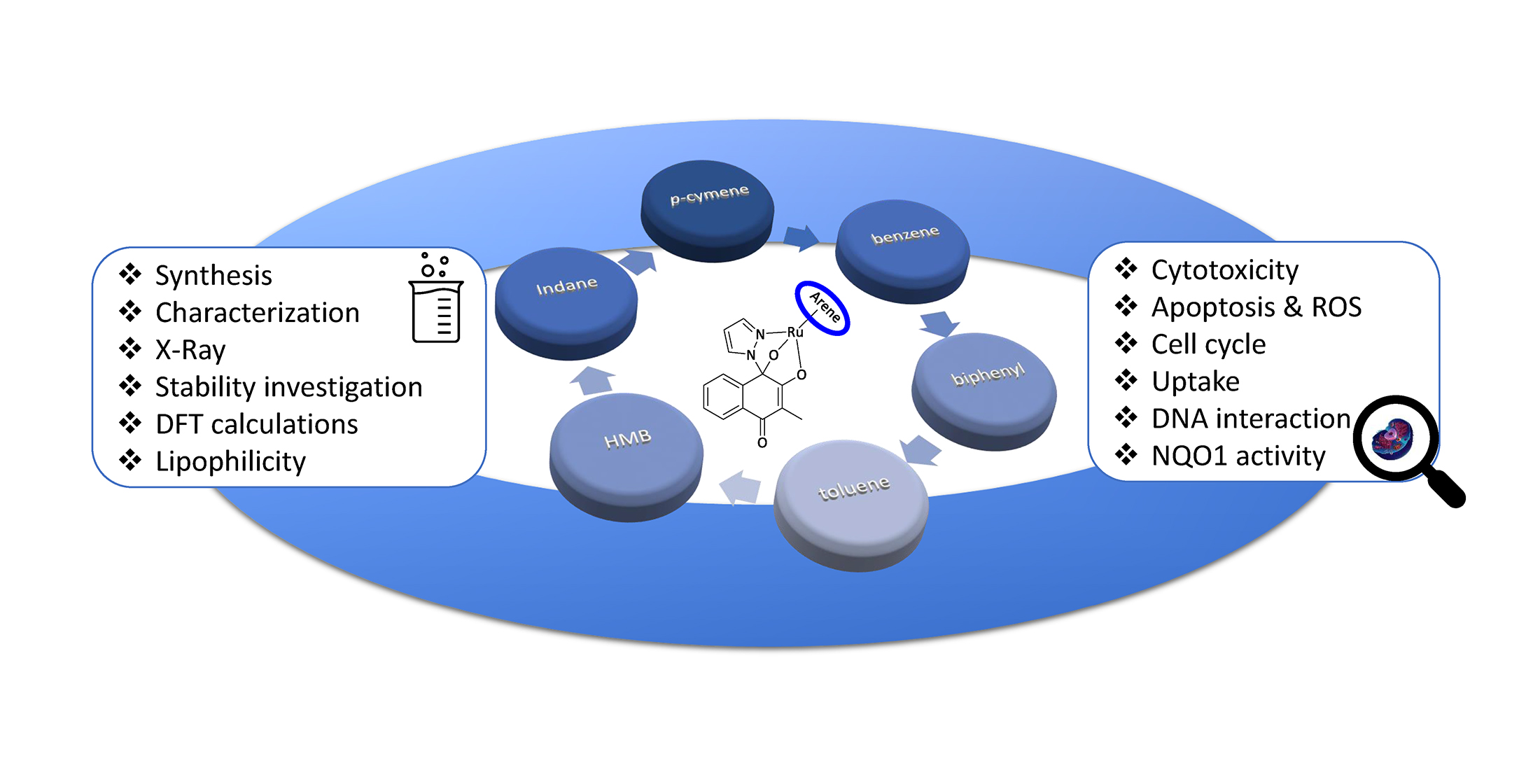
Pharmaceutics, Free Full-Text
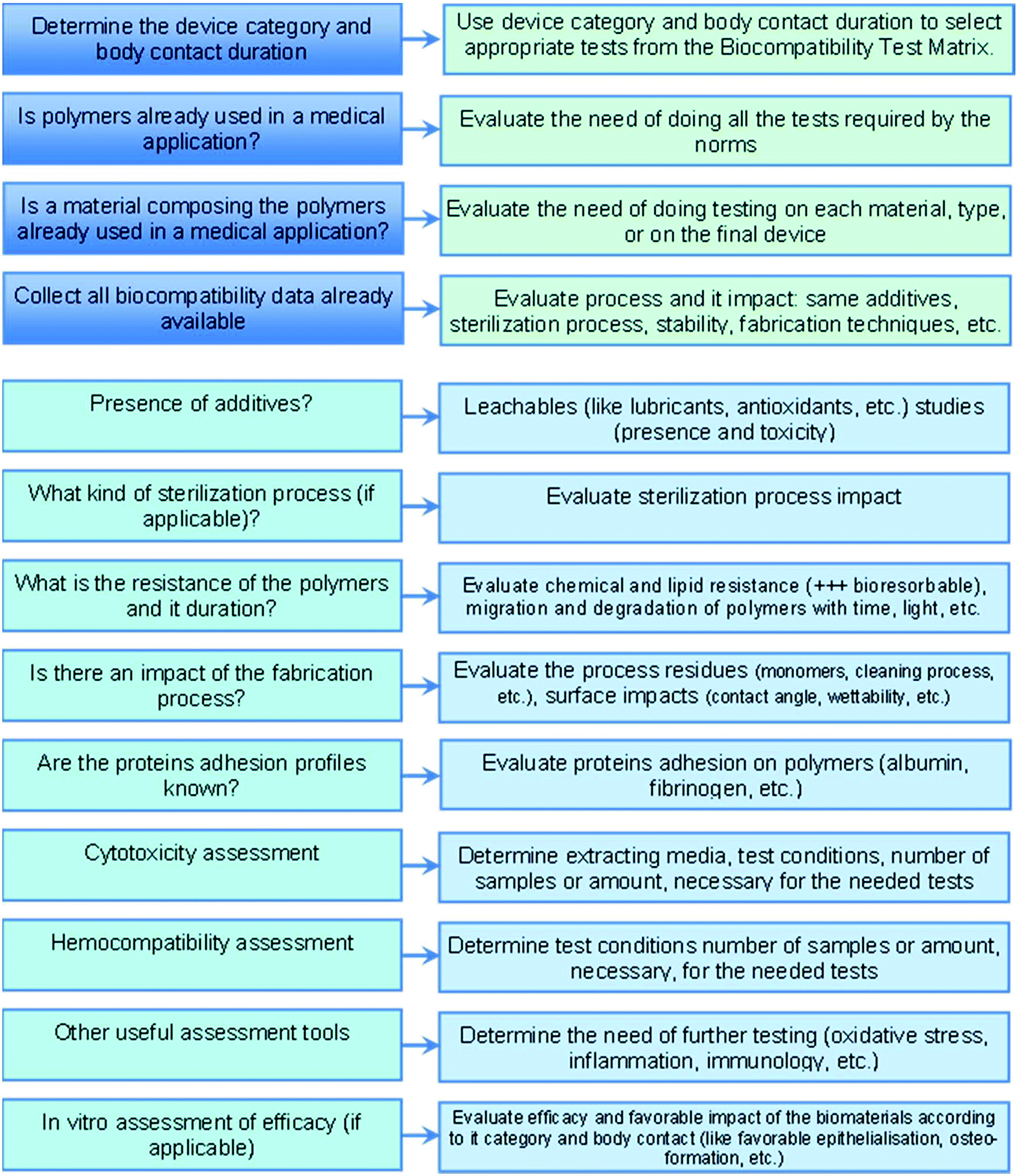
Biocompatibility of polymer-based biomaterials and medical devices – regulations, in vitro screening and risk-management - Biomaterials Science (RSC Publishing) DOI:10.1039/C8BM00518D
Recomendado para você
-
 vivo x90 pro camera zoom test vivo x90 pro zoom test29 março 2025
vivo x90 pro camera zoom test vivo x90 pro zoom test29 março 2025 -
 What is an In Vivo Test?29 março 2025
What is an In Vivo Test?29 março 2025 -
 In Vivo (vs In Vitro and Ex Vivo) - The Definitive Guide29 março 2025
In Vivo (vs In Vitro and Ex Vivo) - The Definitive Guide29 março 2025 -
 Ex vivo testing rig. Download Scientific Diagram29 março 2025
Ex vivo testing rig. Download Scientific Diagram29 março 2025 -
 Vivo Y76 5G Review: Two Pro- and three major cons29 março 2025
Vivo Y76 5G Review: Two Pro- and three major cons29 março 2025 -
 Android 11 Update available for Vivo S1. Vivo V15 Pro (Greyscale Test)29 março 2025
Android 11 Update available for Vivo S1. Vivo V15 Pro (Greyscale Test)29 março 2025 -
 vivo iQOO 12 Pro undergoes AnTuTu test - S2429 março 2025
vivo iQOO 12 Pro undergoes AnTuTu test - S2429 março 2025 -
 Vivo NEX Battery Test: Remarkable Battery Life With Fast Charging Support29 março 2025
Vivo NEX Battery Test: Remarkable Battery Life With Fast Charging Support29 março 2025 -
 Ready-to-use ex vivo human skin models for cosmetic testing29 março 2025
Ready-to-use ex vivo human skin models for cosmetic testing29 março 2025 -
 Vivo X30 Pro has passed Antutu performance test29 março 2025
Vivo X30 Pro has passed Antutu performance test29 março 2025
você pode gostar
-
Genshin Impact & Honkai Impact & Honkai: Star Rail Auto Daily Check-In29 março 2025
-
 Yu Yu Hakusho Minimalist poster, Anime canvas, Anime29 março 2025
Yu Yu Hakusho Minimalist poster, Anime canvas, Anime29 março 2025 -
 CAMISA MAN CITY PRE JOGO II 23 PUMA MASCamisa Pré-Jogo Manchester City II 23 Masculina Puma em Promoção29 março 2025
CAMISA MAN CITY PRE JOGO II 23 PUMA MASCamisa Pré-Jogo Manchester City II 23 Masculina Puma em Promoção29 março 2025 -
 Five Nights at Freddy's Nightmare Fredbear Worm on a String iPad29 março 2025
Five Nights at Freddy's Nightmare Fredbear Worm on a String iPad29 março 2025 -
 Scary Evil Horror Teacher: Scary Prankster 3D Game APK for Android29 março 2025
Scary Evil Horror Teacher: Scary Prankster 3D Game APK for Android29 março 2025 -
 Comicbook movie Rotten Tomatoes score comparison - post - Imgur29 março 2025
Comicbook movie Rotten Tomatoes score comparison - post - Imgur29 março 2025 -
song finder online url29 março 2025
-
 Lassen Volcanic National Park - Trek with Judy29 março 2025
Lassen Volcanic National Park - Trek with Judy29 março 2025 -
 Vivo IPL M49 - KKR v SRH29 março 2025
Vivo IPL M49 - KKR v SRH29 março 2025 -
![Animated] Wulfric 'Sonic' Style Sprite by SvinnValdyr -- Fur](https://d.furaffinity.net/art/svinnvaldyr/1488278723/1342234994.svinnvaldyr_wulfric_sonic_animated_ready_stance.gif) Animated] Wulfric 'Sonic' Style Sprite by SvinnValdyr -- Fur29 março 2025
Animated] Wulfric 'Sonic' Style Sprite by SvinnValdyr -- Fur29 março 2025